11.7 Chemistry of Acid Halides
Preparation of Acid Halides
Acid chlorides are prepared from carboxylic acids by reaction with thionyl chloride (SOCl2), as we saw in the previous section. Similar reaction of a carboxylic acid with phosphorus tribromide (PBr3) yields the acid bromide.

Reactions of Acid Halides
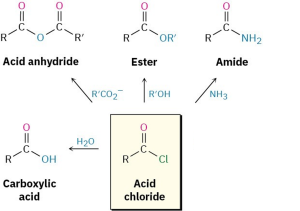
Acid halides are among the most reactive of carboxylic acid derivatives and can be converted into many other kinds of compounds by nucleophilic acyl substitution mechanisms. The halogen can be replaced by –OH to yield an acid, by –OCOR to yield an anhydride, by –OR to yield an ester, by –NH2 to yield an amide, or by R′ to yield a ketone. In addition, the reduction of an acid halide yields a primary alcohol, and reaction with a Grignard reagent yields a tertiary alcohol. Although the reactions we’ll be discussing in this section are illustrated only for acid chlorides, similar processes take place with other acid halides.
Conversion of Acid Halides into Carboxylic Acids: Hydrolysis
Acid chlorides react with water to yield carboxylic acids. This hydrolysis reaction is a typical nucleophilic acyl substitution process and is initiated by attack of water on the acid chloride carbonyl group. The tetrahedral intermediate undergoes elimination of Cl– and loss of H+ to give the product carboxylic acid plus HCl.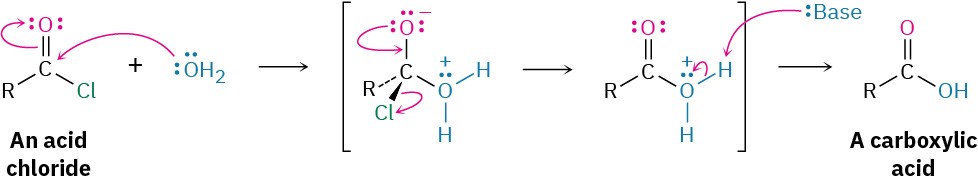 Because HCl is formed during hydrolysis, the reaction is often carried out in the presence of a base such as pyridine or NaOH to remove the HCl and prevent it from causing side reactions.
Because HCl is formed during hydrolysis, the reaction is often carried out in the presence of a base such as pyridine or NaOH to remove the HCl and prevent it from causing side reactions.
Conversion of Acid Halides into Anhydrides
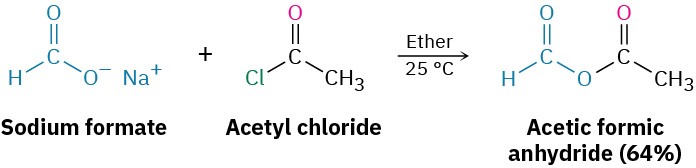
Nucleophilic acyl substitution reaction of an acid chloride with a carboxylate anion gives an acid anhydride. Both symmetrical and unsymmetrical acid anhydrides can be prepared.
Conversion of Acid Halides into Esters: Alcoholysis
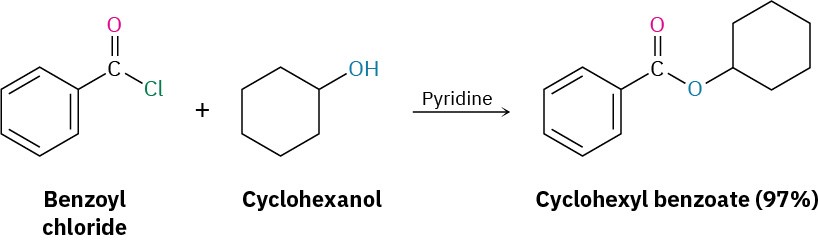
Acid chlorides react with alcohols to yield esters in a process analogous to their reaction with water to yield acids. In fact, this reaction is probably the most common method for preparing esters in the laboratory. As with hydrolysis, alcoholysis reactions are usually carried out in the presence of pyridine or NaOH to react with the HCl formed.
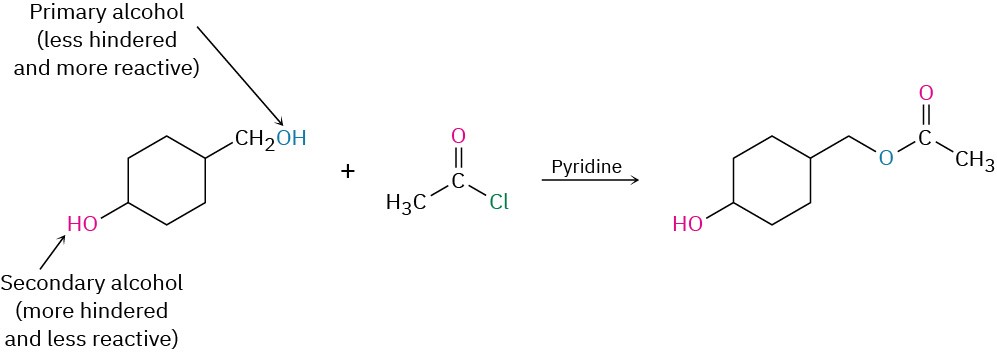
 The reaction of an alcohol with an acid chloride is strongly affected by steric hindrance. Bulky groups on either partner slow down the reaction considerably, resulting in a reactivity order among alcohols of primary > secondary > tertiary. As a result, it’s often possible to selectively esterify an unhindered alcohol in the presence of a more hindered one. This can be important in complex syntheses in which it’s sometimes necessary to distinguish between similar functional groups. For example,
The reaction of an alcohol with an acid chloride is strongly affected by steric hindrance. Bulky groups on either partner slow down the reaction considerably, resulting in a reactivity order among alcohols of primary > secondary > tertiary. As a result, it’s often possible to selectively esterify an unhindered alcohol in the presence of a more hindered one. This can be important in complex syntheses in which it’s sometimes necessary to distinguish between similar functional groups. For example,
 Problem 11.18
Problem 11.18
How might you prepare the following esters using a nucleophilic acyl substitution reaction of an acid chloride?
(a) CH3CH2CO2CH3
(b) CH3CO2CH2CH3
(c) Ethyl benzoate
Problem 11.19
Which method would you choose if you wanted to prepare cyclohexyl benzoate—Fischer esterification or reaction of an acid chloride with an alcohol? Explain.
Conversion of Acid Halides into Amides: Aminolysis
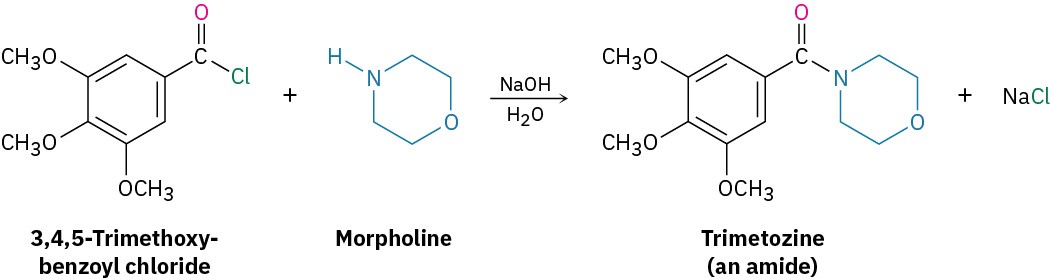
Acid chlorides react rapidly with ammonia and amines to give amides. As with the acid chloride-plus-alcohol method for preparing esters, this reaction of acid chlorides with amines is the most commonly used laboratory method for preparing amides. Both monosubstituted and disubstituted amines can be used, but not trisubstituted amines (R3N).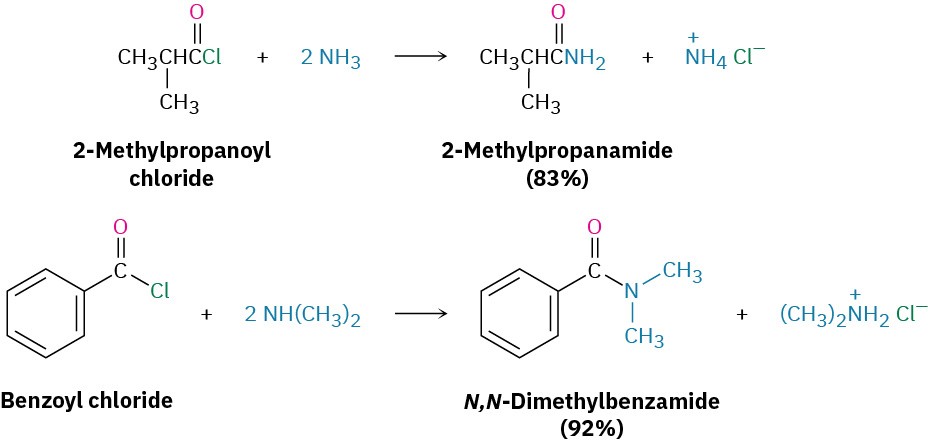 Because HCl is formed during the reaction, two equivalents of the amine must be used. One equivalent reacts with the acid chloride, and one equivalent reacts with the HCl by-product to form an ammonium chloride salt. If, however, the amine component is valuable, amide synthesis is often carried out using one equivalent of the amine plus one equivalent of an inexpensive base such as NaOH. For example, the sedative trimetozine is prepared commercially by reaction of 3,4,5-trimethoxybenzoyl chloride with the amine morpholine in the presence of one equivalent of NaOH.
Because HCl is formed during the reaction, two equivalents of the amine must be used. One equivalent reacts with the acid chloride, and one equivalent reacts with the HCl by-product to form an ammonium chloride salt. If, however, the amine component is valuable, amide synthesis is often carried out using one equivalent of the amine plus one equivalent of an inexpensive base such as NaOH. For example, the sedative trimetozine is prepared commercially by reaction of 3,4,5-trimethoxybenzoyl chloride with the amine morpholine in the presence of one equivalent of NaOH.
 Worked Example 11.3: Synthesizing an Amide from an Acid Chloride
Worked Example 11.3: Synthesizing an Amide from an Acid Chloride
How might you prepare N-methylpropanamide by reaction of an acid chloride with an amine?
Strategy
As its name implies, N-methylpropanamide can be made by reaction of methylamine with the acid chloride of propanoic acid.
Solution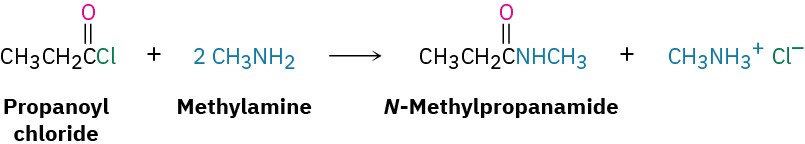 Problem 11.20
Problem 11.20
Write the mechanism of the reaction just shown between 3,4,5-trimethoxybenzoyl chloride and morpholine to form trimetozine. Use curved arrows to show the electron flow in each step.
Problem 11.21
How could you prepare the following amides using an acid chloride and an amine or ammonia?
(a) CH3CH2CONHCH3
(b)N,N-Diethylbenzamide
(c) Propanamide
Conversion of Acid Chlorides into Alcohols: Reduction and Grignard Reaction
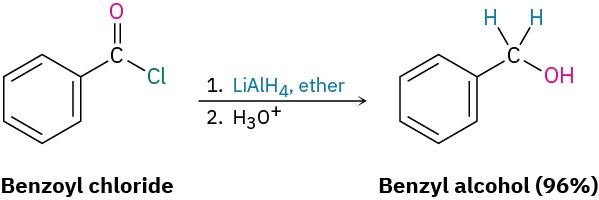
Acid chlorides are reduced by LiAlH4 to yield primary alcohols. The reaction is of little practical value, however, because the parent carboxylic acids are generally more readily available and can themselves be reduced by LiAlH4 to yield alcohols.
Reduction occurs via a typical nucleophilic acyl substitution mechanism in which a hydride ion (H:–) adds to the carbonyl group, yielding a tetrahedral intermediate that expels Cl–.
The net effect is a substitution of –Cl by –H to yield an aldehyde, which is then further reduced by LiAlH4 in a second step to yield the primary alcohol.
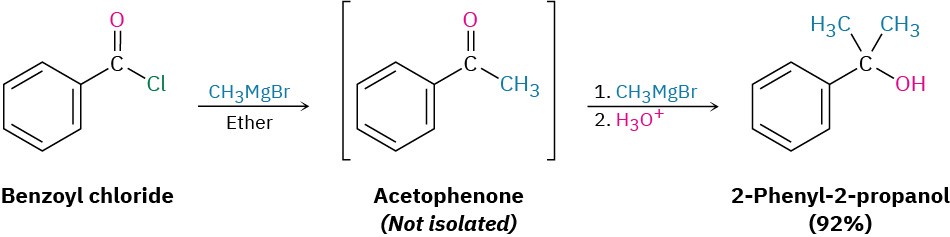
Grignard reagents react with acid chlorides to yield tertiary alcohols with two identical substituents. The mechanism of the reaction is similar to that of LiAlH4 reduction. The first equivalent of Grignard reagent adds to the acid chloride, loss of Cl– from the tetrahedral intermediate yields a ketone, and a second equivalent of Grignard reagent immediately adds to the ketone to produce an alcohol.