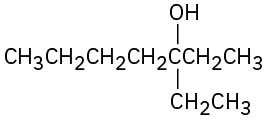11.9 Chemistry of Esters
Preparation of Esters
Esters are usually prepared from carboxylic acids by the methods already discussed. Thus, carboxylic acids are converted directly into esters by SN2 reaction of a carboxylate ion with a primary alkyl halide or by Fischer esterification of a carboxylic acid with an alcohol in the presence of a mineral acid catalyst. In addition, acid chlorides are converted into esters by treatment with an alcohol in the presence of base.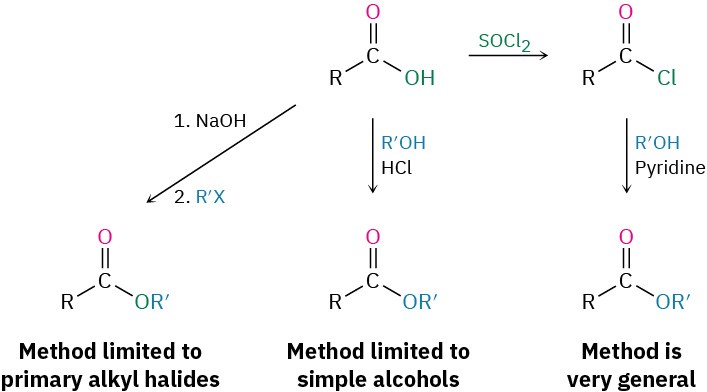
Reactions of Esters
Esters undergo the same kinds of reactions that we’ve seen for other carboxylic acid derivatives, but they are less reactive toward nucleophiles than either acid chlorides or anhydrides. All their reactions are applicable to both acyclic and cyclic esters, called lactones.
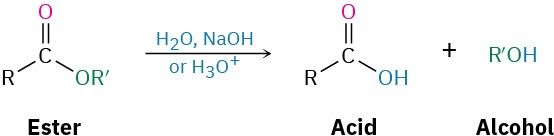
Conversion of Esters into Carboxylic Acids: Hydrolysis
An ester is hydrolyzed, either by aqueous base or aqueous acid, to yield a carboxylic acid plus an alcohol.
 Ester hydrolysis in basic solution is called saponification, after the Latin word sapo, meaning “soap.” As shown in Figure 11.10, ester hydrolysis occurs through a typical nucleophilic acyl substitution pathway in which hydroxide ion is the nucleophile that adds to the ester carbonyl group to give a tetrahedral intermediate. Loss of alkoxide ion then gives a carboxylic acid, which is deprotonated to give the carboxylate ion. Addition of aqueous HCl, in a separate step after the saponification is complete, protonates the carboxylate ion and gives the carboxylic acid.
Ester hydrolysis in basic solution is called saponification, after the Latin word sapo, meaning “soap.” As shown in Figure 11.10, ester hydrolysis occurs through a typical nucleophilic acyl substitution pathway in which hydroxide ion is the nucleophile that adds to the ester carbonyl group to give a tetrahedral intermediate. Loss of alkoxide ion then gives a carboxylic acid, which is deprotonated to give the carboxylate ion. Addition of aqueous HCl, in a separate step after the saponification is complete, protonates the carboxylate ion and gives the carboxylic acid.
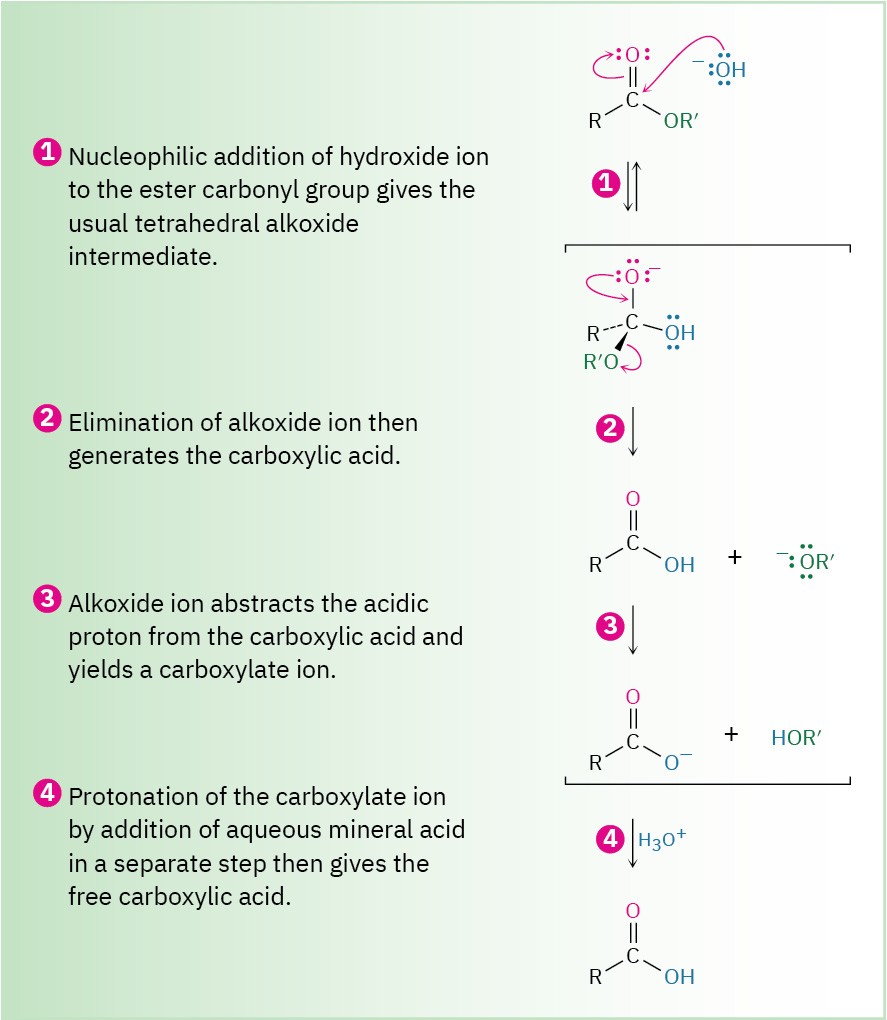
Figure 11.10 MECHANISM: Mechanism of base-induced ester hydrolysis (saponification).
Problem 11.24
Why is the saponification of an ester irreversible? In other words, why doesn’t treatment of a carboxylic acid with an alkoxide ion yield an ester?
Conversion of Esters into Amides: Aminolysis
Esters react with ammonia and amines to yield amides. The reaction is not often used, however, because it’s usually easier to prepare an amide by starting with an acid chloride.
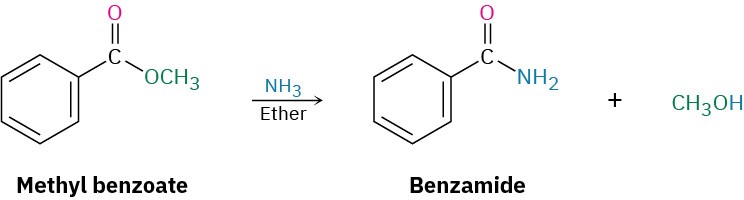
Conversion of Esters into Alcohols: Reduction
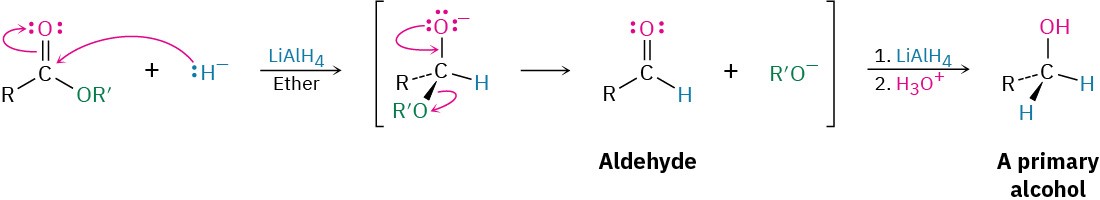
Esters are easily reduced by treatment with LiAlH4 to yield primary alcohols (Section 9.5).
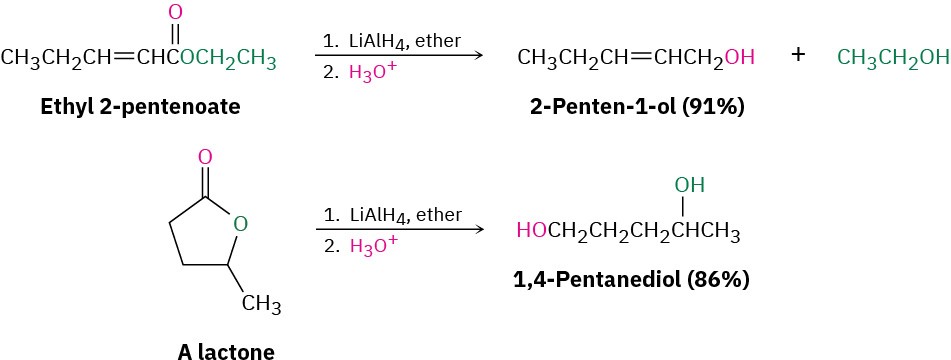
The mechanism of ester reduction is similar to that of acid chloride reduction in that a hydride ion first adds to the carbonyl group, followed by elimination of alkoxide ion to yield an aldehyde. Further reduction of the aldehyde gives the primary alcohol.
 Problem 11.25
Problem 11.25
What product would you expect from the reaction of butyrolactone with LiAlH4?

Problem 11.26
Show the products you would obtain by reduction of the following esters with LiAlH4:
(a)
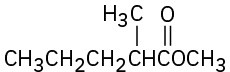
(b)
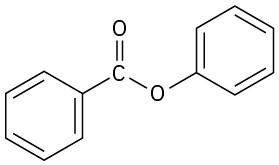
Conversion of Esters into Alcohols: Grignard Reaction
Esters react with 2 equivalents of a Grignard reagent to yield a tertiary alcohol in which two of the substituents are identical (Section 9.6). The reaction occurs by the usual nucleophilic substitution mechanism to give an intermediate ketone, which reacts further with the Grignard reagent to yield a tertiary alcohol.
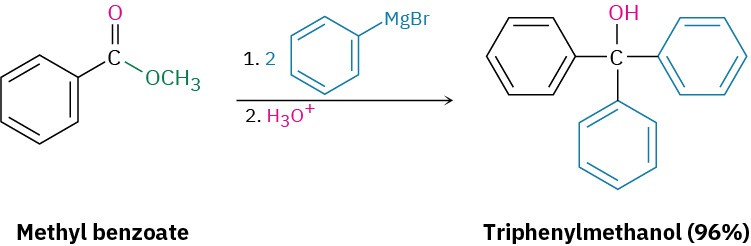
Problem 11.27
What ester and what Grignard reagent might you start with to prepare the following alcohols?
(a)
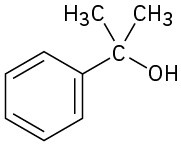
(b)
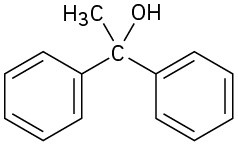
(c)