2.8 Conformations of Other Alkanes
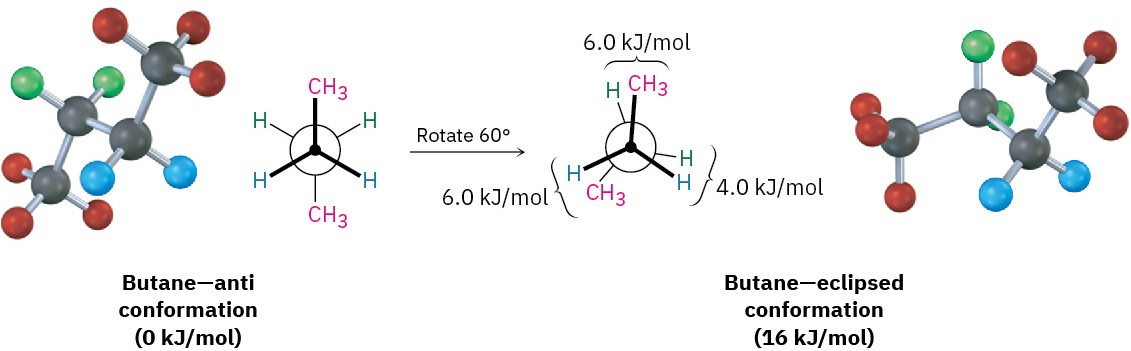
The conformational situation becomes more complex for larger alkanes because not all staggered conformations have the same energy and not all eclipsed conformations have the same energy. In butane, for instance, the lowest-energy arrangement, called the anti conformation, is the one in which the two methyl groups are as far apart as possible—180° away from each other. As rotation around the C2–C3 bond occurs, an eclipsed conformation is reached where there are two CH3 ⟷ H interactions and one H ⟷ H interaction.
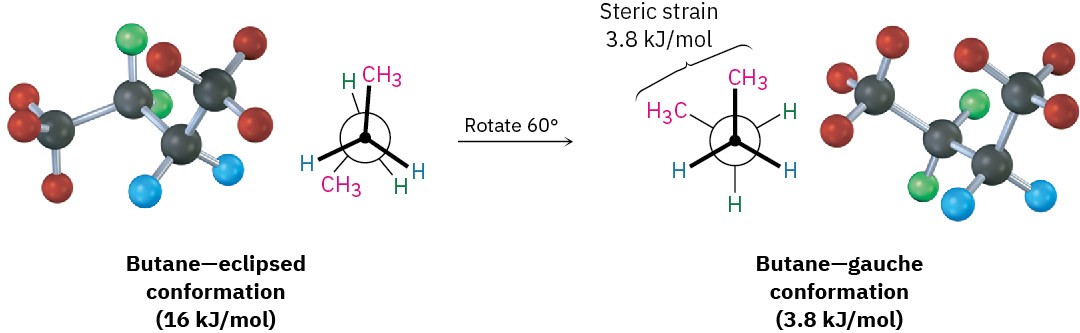
 As bond rotation continues, an energy minimum is reached at the staggered conformation where the methyl groups are 60° apart. Called the gauche conformation, it lies 3.8 kJ/mol (0.9 kcal/mol) higher in energy than the anti conformation even though it has no eclipsing interactions. This energy difference occurs because the hydrogen atoms of the methyl groups are near one another in the gauche conformation, resulting in what is called steric strain. Steric strain is the repulsive interaction that occurs when atoms are forced closer together than their atomic radii allow. It’s the result of trying to force two atoms to occupy the same space.
As bond rotation continues, an energy minimum is reached at the staggered conformation where the methyl groups are 60° apart. Called the gauche conformation, it lies 3.8 kJ/mol (0.9 kcal/mol) higher in energy than the anti conformation even though it has no eclipsing interactions. This energy difference occurs because the hydrogen atoms of the methyl groups are near one another in the gauche conformation, resulting in what is called steric strain. Steric strain is the repulsive interaction that occurs when atoms are forced closer together than their atomic radii allow. It’s the result of trying to force two atoms to occupy the same space.
 A plot of potential energy versus rotation about the C2-C3 bond is shown in Figure 2.10.
A plot of potential energy versus rotation about the C2-C3 bond is shown in Figure 2.10. 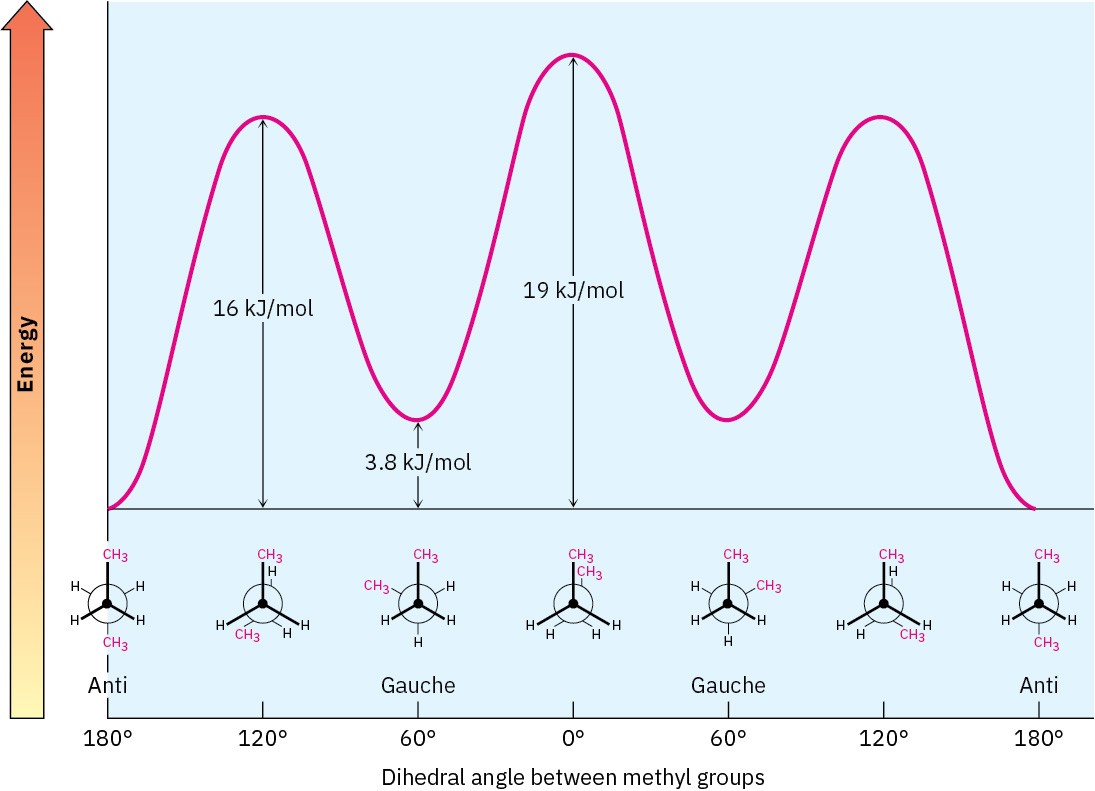 Figure 2.10 A plot of potential energy versus rotation for the C2−C3 bond in butane. The energy maximum occurs when the two methyl groups eclipse each other, and the energy minimum occurs when the two methyl groups are 180° apart (anti).
Figure 2.10 A plot of potential energy versus rotation for the C2−C3 bond in butane. The energy maximum occurs when the two methyl groups eclipse each other, and the energy minimum occurs when the two methyl groups are 180° apart (anti).
The same principles just developed for butane apply to pentane, hexane, and all higher alkanes. The most favorable conformation for any alkane has the carbon–carbon bonds in staggered arrangements, with large substituents arranged anti to one another. 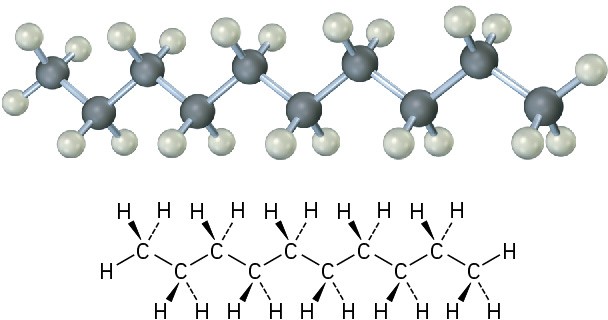 Figure 2.11 The most stable alkane conformation is the one in which all substituents are staggered and the carbon–carbon bonds are arranged anti, as shown in this model of decane.
Figure 2.11 The most stable alkane conformation is the one in which all substituents are staggered and the carbon–carbon bonds are arranged anti, as shown in this model of decane.
One final point: saying that one particular conformer is “more stable” than another doesn’t mean the molecule adopts and maintains only the more stable conformation. At room temperature, rotations around σ bonds occur so rapidly that all conformers are in equilibrium. At any given instant, however, a larger percentage of molecules will be found in a more stable conformation than in a less stable one.
Worked Example 2.4: Newman Projections
Sight along the C1–C2 bond of 1-chloropropane, and draw Newman projections of the most stable and least stable conformations.
Strategy
The most stable conformation of a substituted alkane is generally a staggered one in which large groups have an anti relationship. The least stable conformation is generally an eclipsed one in which large groups are as close as possible.
Solution
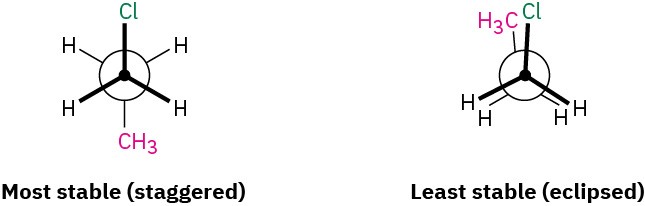 Problem 2.18
Problem 2.18
Sight along the C2–C1 bond of 2-methylpropane (isobutane).
(a) Draw a Newman projection of the most stable conformation.
(b) Draw a Newman projection of the least stable conformation.
Problem 2.19
Sight along the C2–C3 bond of 2,3-dimethylbutane, and draw a Newman projection of the most stable conformation.
Problem 2.20
Draw a Newman projection along the C2–C3 bond of the following conformation of 2,3- dimethylbutane.