9.13 Cyclic Ethers: Epoxides
For the most part, cyclic ethers behave like acyclic ethers. The chemistry of the ether functional group is the same, whether it’s in an open chain or in a ring. Common cyclic ethers such as tetrahydrofuran and dioxane are often used as solvents because of their inertness, yet they can be cleaved by strong acids.

The one group of cyclic ethers that behaves differently from open-chain ethers are the three-membered-ring compounds called epoxides, or oxiranes, which we saw in Section 5.6. The strain of the three-membered ring gives epoxides unique chemical reactivity.
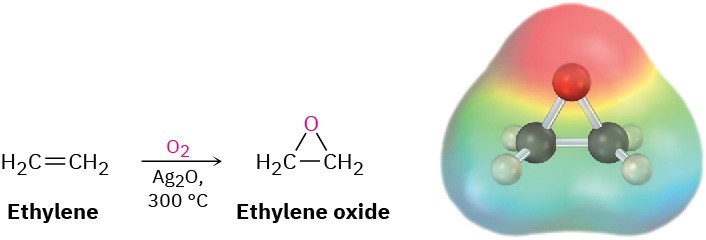
Ethylene oxide, the simplest epoxide, is an intermediate in the manufacture of both ethylene glycol, used for automobile antifreeze, and polyester polymers. Approximately 37 million tons of ethylene oxide is produced worldwide each year, most of it by air oxidation of ethylene over a silver oxide catalyst at 300 °C. This process is not useful for other epoxides, however, and is of little value in the laboratory.
Note that the name ethylene oxide is not a systematic one because the –ene ending implies the presence of a double bond in the molecule. The name is frequently used, however, because ethylene oxide is derived from ethylene by addition of an oxygen atom. Other simple epoxides are named similarly. The systematic name for ethylene oxide is 1,2- epoxyethane.
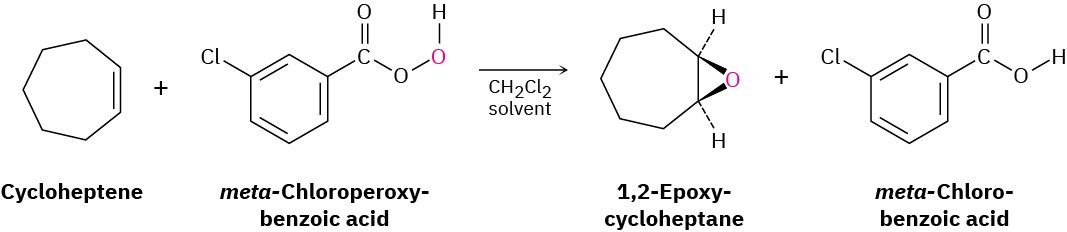
In the laboratory, as we saw in Section 5.6, epoxides are usually prepared by treatment of an alkene with a peroxyacid (RCO3H), typically m-chloroperoxybenzoic acid (mCPBA).
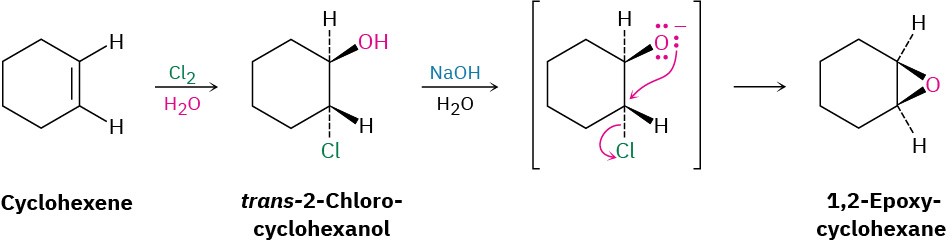
Epoxides can also be prepared from halohydrins, themselves produced by electrophilic addition of Cl2 or Br2 to an alkene in the presence of water (Section 5.5). When a halohydrin is treated with base, HX is eliminated and an epoxide is produced by an intramolecular Williamson ether synthesis. That is, the nucleophilic alkoxide ion and the electrophilic alkyl halide are in the same molecule.
 Problem 9.20
Problem 9.20
What product would you expect from reaction of trans-2-butene with m-chloroperoxybenzoic acid? Show the stereochemistry.
Problem 9.21
Reaction of cis-2-butene with m-chloroperoxybenzoic acid yields an epoxide different from that obtained by reaction of the trans isomer. Explain.

