5.9 Electrophilic Additions to Conjugated Dienes: Allylic Carbocations
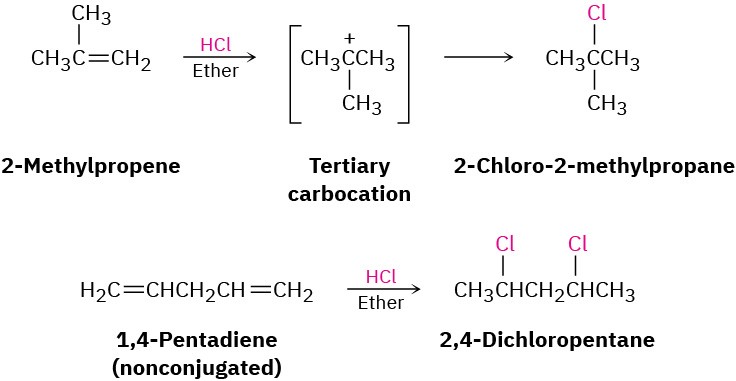
One of the most striking differences between conjugated dienes and typical alkenes is their behavior in electrophilic addition reactions. To review briefly, the addition of an electrophile to a carbon–carbon double bond is a general reaction of alkenes (Section 5.1). Markovnikov regiochemistry is observed because the more stable carbocation is formed as an intermediate. Thus, addition of HCl to 2-methylpropene yields 2-chloro-2-methylpropane rather than 1-chloro-2-methylpropane, and addition of 2 equivalents of HCl to the nonconjugated diene 1,4-pentadiene yields 2,4-dichloropentane.
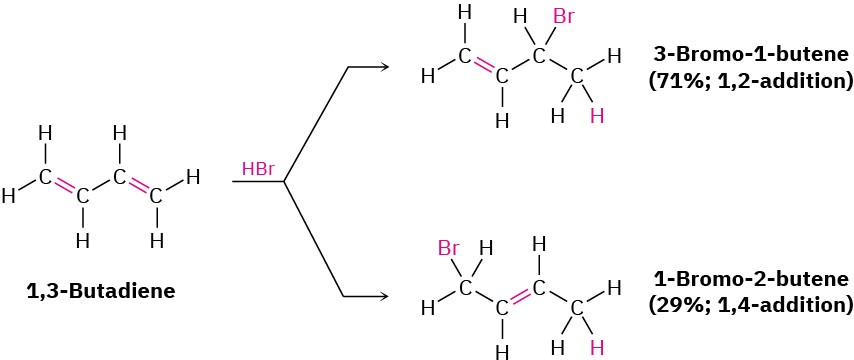
 Conjugated dienes also undergo electrophilic addition reactions readily, but mixtures of products are invariably obtained. Addition of HBr to 1,3-butadiene, for instance, yields a mixture of two products (not counting cis–trans isomers). 3-Bromo-1-butene is the typical Markovnikov product of 1,2-addition to a double bond, but 1-bromo-2-butene seems unusual. The double bond in this product has moved to a position between carbons 2 and 3, and HBr has added to carbons 1 and 4, a result described as 1,4-addition.
Conjugated dienes also undergo electrophilic addition reactions readily, but mixtures of products are invariably obtained. Addition of HBr to 1,3-butadiene, for instance, yields a mixture of two products (not counting cis–trans isomers). 3-Bromo-1-butene is the typical Markovnikov product of 1,2-addition to a double bond, but 1-bromo-2-butene seems unusual. The double bond in this product has moved to a position between carbons 2 and 3, and HBr has added to carbons 1 and 4, a result described as 1,4-addition.
 Many other electrophiles besides HBr add to conjugated dienes, and mixtures of products are usually formed. For example, Br2 adds to 1,3-butadiene to give a mixture of 3,4- dibromo-1-butene and 1,4-dibromo-2-butene.
Many other electrophiles besides HBr add to conjugated dienes, and mixtures of products are usually formed. For example, Br2 adds to 1,3-butadiene to give a mixture of 3,4- dibromo-1-butene and 1,4-dibromo-2-butene.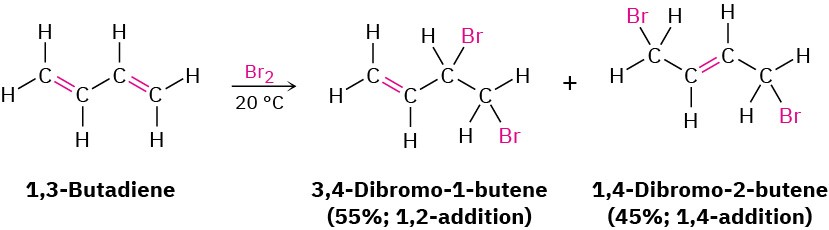 How can we account for the formation of 1,4-addition products? The answer is that allylic carbocations are involved as intermediates (recall that the word allylic means “next to a double bond”). When 1,3-butadiene reacts with an electrophile such as H+, two carbocation intermediates are possible—a primary nonallylic carbocation and a secondary allylic cation. Because an allylic cation is stabilized by resonance between two forms (Section 1.15), it is more stable and forms faster than a nonallylic carbocation.
How can we account for the formation of 1,4-addition products? The answer is that allylic carbocations are involved as intermediates (recall that the word allylic means “next to a double bond”). When 1,3-butadiene reacts with an electrophile such as H+, two carbocation intermediates are possible—a primary nonallylic carbocation and a secondary allylic cation. Because an allylic cation is stabilized by resonance between two forms (Section 1.15), it is more stable and forms faster than a nonallylic carbocation.
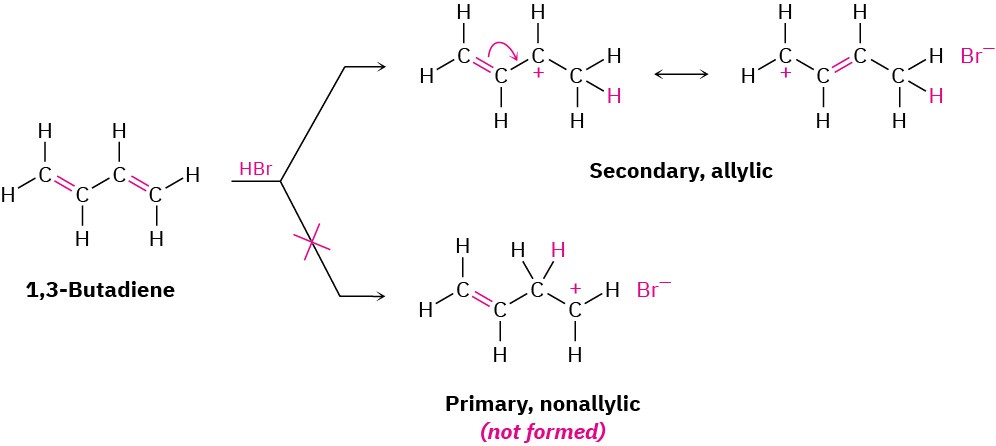 When the allylic cation reacts with Br– to complete the electrophilic addition, the reaction can occur either at C1 or at C3 because both carbons share the positive charge (Figure 5.12). Thus, a mixture of 1,2- and 1,4-addition products results.
When the allylic cation reacts with Br– to complete the electrophilic addition, the reaction can occur either at C1 or at C3 because both carbons share the positive charge (Figure 5.12). Thus, a mixture of 1,2- and 1,4-addition products results.
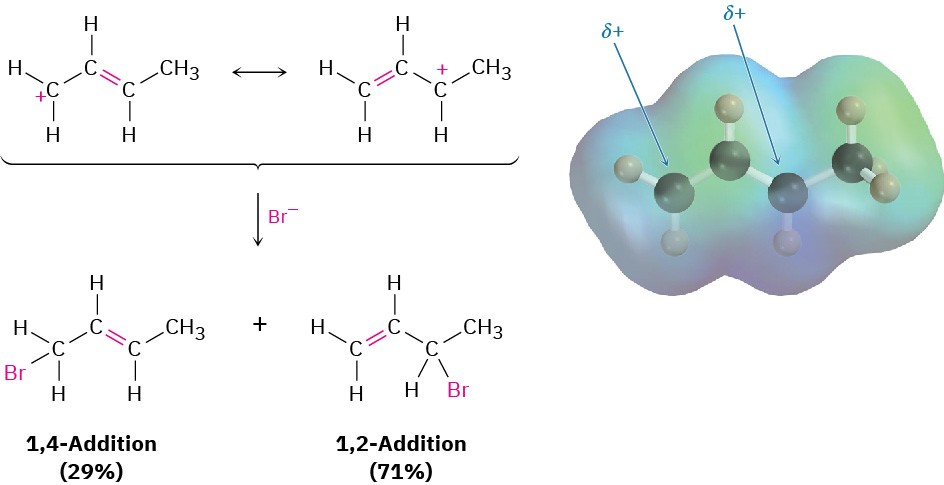 Figure 5.12 An electrostatic potential map of the allylic carbocation produced by protonation of 1,3-butadiene shows that the positive charge is shared by carbons 1 and 3. Reaction of Br– with the more positive carbon (C3) predominantly yields the 1,2- addition product.
Figure 5.12 An electrostatic potential map of the allylic carbocation produced by protonation of 1,3-butadiene shows that the positive charge is shared by carbons 1 and 3. Reaction of Br– with the more positive carbon (C3) predominantly yields the 1,2- addition product.
Worked Example 5.4: Predicting the Product of an Electrophilic Addition Reaction of a Conjugated Diene
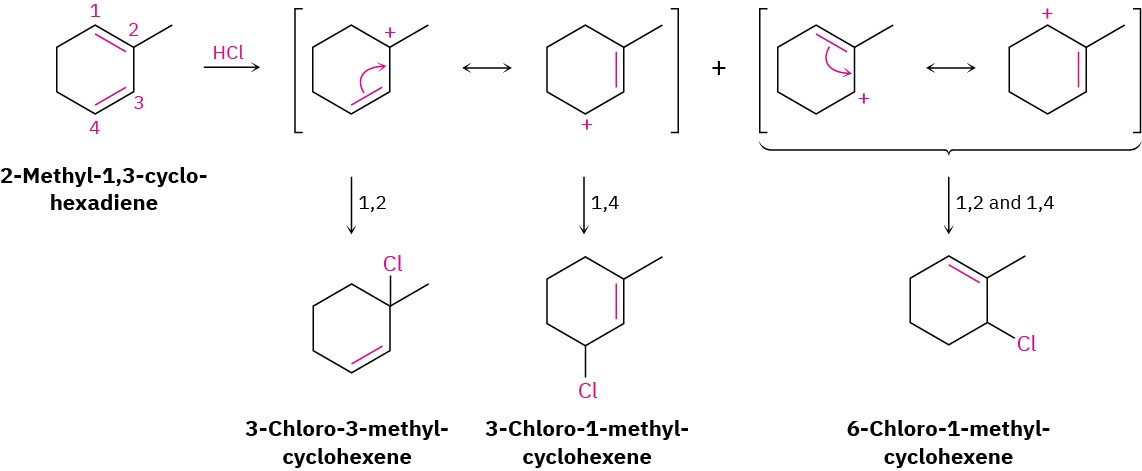
Give the structures of the likely products from reaction of 1 equivalent of HCl with 2- methyl-1,3-cyclohexadiene. Show both 1,2 and 1,4 adducts.
Strategy
Electrophilic addition of HCl to a conjugated diene involves the formation of allylic carbocation intermediate. Thus, the first step is to protonate the two ends of the diene and draw the resonance forms of the two allylic carbocations that result. Then, allow each resonance form to react with Cl–, generating a maximum of four possible products.In the present instance, protonation of the C1–C2 double bond gives a carbocation that can react further to give the 1,2 adduct 3-chloro-3-methylcyclohexene and the 1,4 adduct 3- chloro-1-methylcyclohexene. Protonation of the C3–C4 double bond gives a symmetrical carbocation, whose two resonance forms are equivalent. Thus, the 1,2 adduct and the 1,4 adduct have the same structure: 6-chloro-1-methylcyclohexene. Of the two possible modes of protonation, the first is more likely because it yields a more stable, tertiary allylic cation rather than a less-stable, secondary allylic cation.
Solution
Problem 5.9
Give the structures of both 1,2 and 1,4 adducts resulting from reaction of 1 equivalent of HCl with 1,3-pentadiene.
Problem 5.10
Look at the possible carbocation intermediates produced during addition of HCl to 1,3-pentadiene (Problem 5.9), and predict which 1,2 adduct predominates. Which 1,4 adduct predominates?
Problem 5.11
Give the structures of both 1,2 and 1,4 adducts resulting from reaction of 1 equivalent of HBr with the following compound: