4.6 How Organic Reactions Occur: Mechanisms
Having looked at the kinds of reactions that take place, let’s now see how they occur. An overall description of how a reaction occurs is called a reaction mechanism. A mechanism describes in detail exactly what takes place at each stage of a chemical transformation— which bonds are broken and in what order, which bonds are formed and in what order, and what the relative rates are for each step. A complete mechanism must also account for all reactants used and all products formed.
All chemical reactions involve bond-breaking and bond-making. When two molecules come together, react, and yield products, specific bonds in the reactant molecules are broken and specific bonds in the product molecules are formed. Fundamentally, there are two ways in which a covalent two-electron bond can break. A bond can break in an electronically unsymmetrical way so that both bonding electrons remain with one product fragment, leaving the other with a vacant orbital, or a bond can break in an electronically symmetrical way so that one electron remains with each product fragment. The unsymmetrical cleavage is said to be heterolytic, and the symmetrical cleavage is said to be homolytic.
We’ll develop this point in more detail later, but note for now that the movement of two electrons in the unsymmetrical process is indicated using a full-headed curved arrow (![]() ), whereas the movement of one electron in the symmetrical process is indicated using a half- headed, or “fishhook,” arrow (
), whereas the movement of one electron in the symmetrical process is indicated using a half- headed, or “fishhook,” arrow (![]() ).
).

Just as there are two ways in which a bond can break, there are two ways in which a covalent two-electron bond can form. A bond can form in an electronically unsymmetrical way if both bonding electrons are donated to the new bond by one reactant, or in a symmetrical way if one electron is donated by each reactant.
 Processes that involve unsymmetrical bond-breaking and bond-making are called polar reactions. Polar reactions involve species that have an even number of electrons and thus have only electron pairs in their orbitals. Polar processes are by far the more common reaction type in both organic and biological chemistry, and a large part of this book is devoted to their description.
Processes that involve unsymmetrical bond-breaking and bond-making are called polar reactions. Polar reactions involve species that have an even number of electrons and thus have only electron pairs in their orbitals. Polar processes are by far the more common reaction type in both organic and biological chemistry, and a large part of this book is devoted to their description.
Processes that involve symmetrical bond-breaking and bond-making are called radical reactions. A radical, often called a free radical, is a neutral chemical species that contains an odd number of electrons and thus has a single, unpaired electron in one of its orbitals.
Polar reactions occur because of the electrical attraction between positively polarized and negatively polarized centers on functional groups in molecules. To see how these reactions take place, let’s first recall the discussion of polar covalent bonds in Section 1.12 and then look more deeply into the effects of bond polarity on organic molecules.
Most organic compounds are electrically neutral; they have no net charge, either positive or negative. We saw in Section 1.12, however, that certain bonds within a molecule, particularly the bonds in functional groups, are polar. Bond polarity is a consequence of an unsymmetrical electron distribution in a bond and is due to the difference in electronegativity of the bonded atoms.
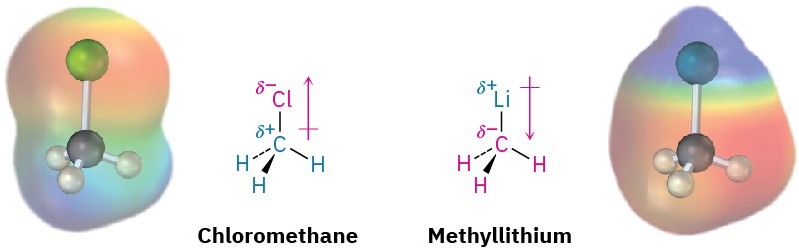
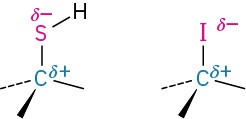
Elements such as oxygen, nitrogen, fluorine, and chlorine are more electronegative than carbon, so a carbon atom bonded to one of these atoms has a partial positive charge (δ+). Metals are less electronegative than carbon, so a carbon atom bonded to a metal has a partial negative charge (δ−). Electrostatic potential maps of chloromethane and methyllithium illustrate these charge distributions, showing that the carbon atom in chloromethane is electron-poor (blue) while the carbon in methyllithium is electron-rich (red).
 The polarity patterns of some common functional groups are shown in Table 4.2. Note that carbon is always positively polarized except when bonded to a metal.
The polarity patterns of some common functional groups are shown in Table 4.2. Note that carbon is always positively polarized except when bonded to a metal.
Table 4.2 Polarity Patterns in Some Common Functional Groups
|
Compound type |
Functional group structure |
| alcohol | 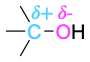 |
| Alkene |  Symmetric, nonpolar Symmetric, nonpolar |
| Alkyl halide | 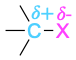 |
| Amine | 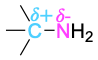 |
| Ether | 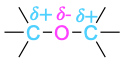 |
| Thiol | 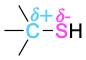 |
| Nitrile | |
| Grignard reagent | 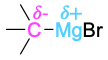 |
| Alkyllithium | 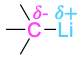 |
| Carbonyl | 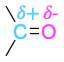 |
| Carboxylic acid | 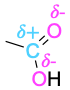 |
| Carboxylic acid chloride | 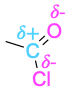 |
| Thioester | 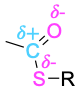 |
| Aldehyde | 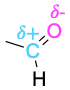 |
| Ester | 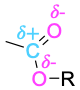 |
| Ketone | 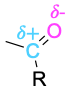 |
This discussion of bond polarity is oversimplified in that we’ve considered only bonds that are inherently polar due to differences in electronegativity. Polar bonds can also result from the interaction of functional groups with acids or bases. Take an alcohol such as methanol, for example. In neutral methanol, the carbon atom is somewhat electron-poor because the electronegative oxygen attracts the electrons in the C−O bond. On protonation of the methanol oxygen by an acid, however, a full positive charge on oxygen attracts the electrons in the C−O bond much more strongly and makes the carbon much more electron- poor. We’ll see numerous examples throughout this book of reactions that are catalyzed by acids because of the resultant increase in bond polarity upon protonation.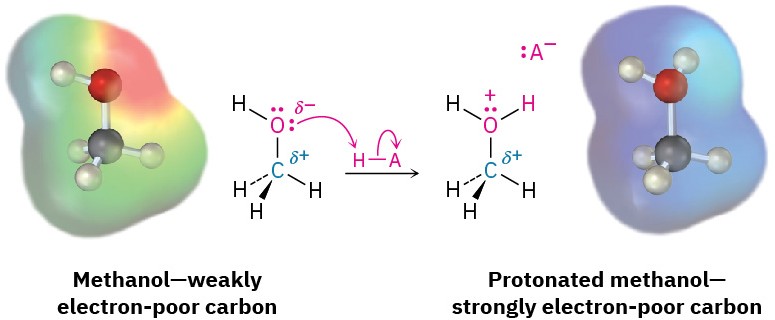
 What does functional-group polarity mean with respect to chemical reactivity? Because unlike charges attract, the fundamental characteristic of all polar organic reactions is that electron-rich sites react with electron-poor sites. Bonds are made when an electron-rich atom donates a pair of electrons to an electron-poor atom, and bonds are broken when one atom leaves with both electrons from the former bond.
What does functional-group polarity mean with respect to chemical reactivity? Because unlike charges attract, the fundamental characteristic of all polar organic reactions is that electron-rich sites react with electron-poor sites. Bonds are made when an electron-rich atom donates a pair of electrons to an electron-poor atom, and bonds are broken when one atom leaves with both electrons from the former bond.
As we saw in Section 1.21, the movement of an electron pair during a polar reaction is indicated using a curved, full-headed arrow to show where electrons move when reactant bonds are broken and product bonds are formed during the reaction.
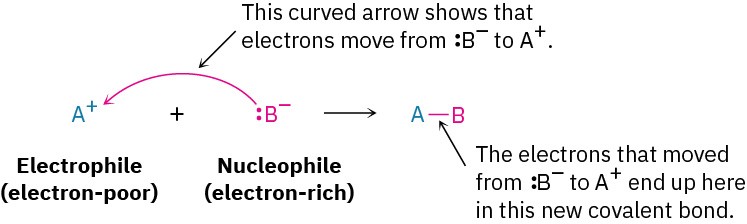 In referring to the electron-rich and electron-poor species involved in polar reactions, chemists use the words nucleophile and electrophile. A nucleophile is a substance that is “nucleus-loving.” (Remember that a nucleus is positively charged.) A nucleophile has a negatively polarized, electron-rich atom and can form a bond by donating a pair of electrons to a positively polarized, electron-poor atom. Nucleophiles can be either neutral or negatively charged; ammonia, water, hydroxide ion, and chloride ion are examples. An electrophile, by contrast, is “electron-loving.” An electrophile has a positively polarized, electron-poor atom and can form a bond by accepting a pair of electrons from a nucleophile. Electrophiles can be either neutral or positively charged. Acids (H+ donors), alkyl halides, and carbonyl compounds are examples (Figure 4.5).
In referring to the electron-rich and electron-poor species involved in polar reactions, chemists use the words nucleophile and electrophile. A nucleophile is a substance that is “nucleus-loving.” (Remember that a nucleus is positively charged.) A nucleophile has a negatively polarized, electron-rich atom and can form a bond by donating a pair of electrons to a positively polarized, electron-poor atom. Nucleophiles can be either neutral or negatively charged; ammonia, water, hydroxide ion, and chloride ion are examples. An electrophile, by contrast, is “electron-loving.” An electrophile has a positively polarized, electron-poor atom and can form a bond by accepting a pair of electrons from a nucleophile. Electrophiles can be either neutral or positively charged. Acids (H+ donors), alkyl halides, and carbonyl compounds are examples (Figure 4.5).
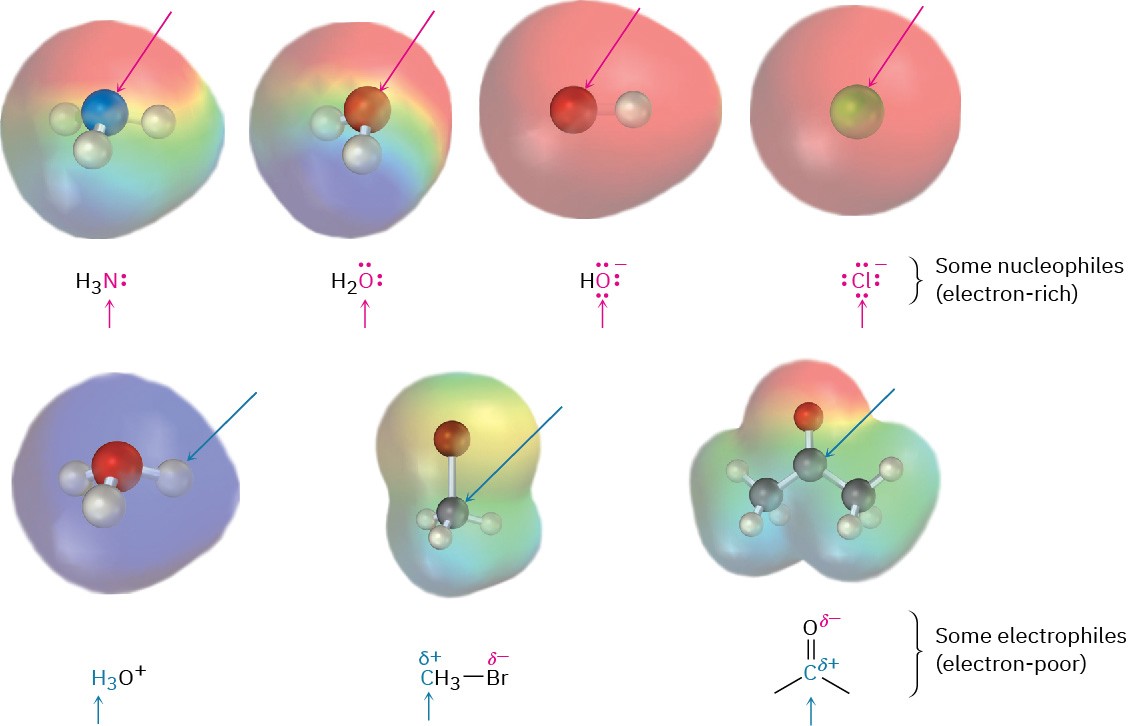 Figure 4.5 Some nucleophiles and electrophiles. Electrostatic potential maps identify the nucleophilic (negative) and electrophilic (positive) atoms.
Figure 4.5 Some nucleophiles and electrophiles. Electrostatic potential maps identify the nucleophilic (negative) and electrophilic (positive) atoms.
Note that neutral compounds can often react either as nucleophiles or as electrophiles, depending on the circumstances. After all, if a compound is neutral yet has an electron-rich nucleophilic site, it must also have a corresponding electron-poor electrophilic site. Water, for instance, acts as an electrophile when it donates H+ but acts as a nucleophile when it donates a nonbonding pair of electrons. Similarly, a carbonyl compound acts as an electrophile when it reacts at its positively polarized carbon atom, yet acts as a nucleophile when it reacts at its negatively polarized oxygen atom.
If the definitions of nucleophiles and electrophiles sound similar to those given in Section 1.21 for Lewis acids and Lewis bases, that’s because there is indeed a correlation. Lewis bases are electron donors and behave as nucleophiles, whereas Lewis acids are electron acceptors and behave as electrophiles. Thus, much of organic chemistry is explainable in terms of acid–base reactions. The main difference is that the words acid and base are used broadly in all fields of chemistry, while the words nucleophile and electrophile are used primarily in organic chemistry when carbon bonding is involved.
Worked Example 4.2: Identifying Electrophiles and Nucleophiles
Which of the following species is likely to behave as a nucleophile and which as an electrophile?
(a) NO2+
(b) CN−
(c) CH3NH2
(d) (CH3)3S+
Strategy
A nucleophile has an electron-rich site, either because it is negatively charged or because it has a functional group containing an atom that has a lone pair of electrons. An electrophile has an electron-poor site, either because it is positively charged or because it has a functional group containing an atom that is positively polarized.
Solution
(a) NO2+ (nitronium ion) is likely to be an electrophile because it is positively charged.
(b) :C≡N– (cyanide ion) is likely to be a nucleophile because it is negatively charged.
(c) CH3NH2 (methylamine) might be either a nucleophile or an electrophile, depending on the circumstances. The lone pair of electrons on the nitrogen atom makes methylamine a potential nucleophile, while positively polarized N−H hydrogens make methylamine a potential acid (electrophile).
(d) (CH3)3S+ (trimethylsulfonium ion) is likely to be an electrophile because it is positively charged.
Problem 4.14
Which of the following species are likely to be nucleophiles and which electrophiles? Which might be both?
(a) CH3Cl
(b) CH3S–
(c)
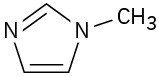
(d)
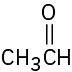
Problem 4.15
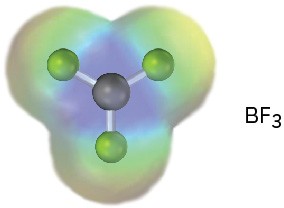
An electrostatic potential map of boron trifluoride is shown. Is BF3 likely to be a nucleophile or an electrophile? Draw a Lewis structure for BF3, and explain your answer.