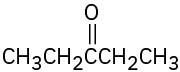
5.11 Hydration of Alkynes
Mercury(II)-Catalyzed Hydration of Alkynes
Alkynes don’t react directly with aqueous acid but will undergo hydration readily in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the −OH group adds to the more highly substituted carbon and the −H attaches to the less highly substituted one.
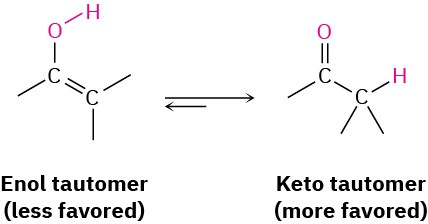
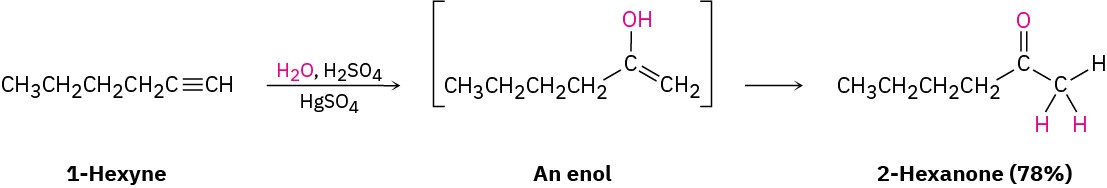 Interestingly, the actual product isolated from alkyne hydration is not a vinylic alcohol, or enol (ene + ol), but is instead a ketone. Although the enol is an intermediate in the reaction, it immediately rearranges into a ketone by a process called keto–enol tautomerism. The individual keto and enol forms are said to be tautomers, a word used to describe two isomers that undergo spontaneous interconversion accompanied by the change in position of a hydrogen. With few exceptions, the keto–enol tautomeric equilibrium lies on the side of the ketone; enols are almost never isolated.
Interestingly, the actual product isolated from alkyne hydration is not a vinylic alcohol, or enol (ene + ol), but is instead a ketone. Although the enol is an intermediate in the reaction, it immediately rearranges into a ketone by a process called keto–enol tautomerism. The individual keto and enol forms are said to be tautomers, a word used to describe two isomers that undergo spontaneous interconversion accompanied by the change in position of a hydrogen. With few exceptions, the keto–enol tautomeric equilibrium lies on the side of the ketone; enols are almost never isolated.
 A mixture of both possible ketones results when an unsymmetrically substituted internal alkyne (RC≡CR’) is hydrated. The reaction is therefore most useful when applied to a terminal alkyne (RC≡CH) because only a methyl ketone is formed.
A mixture of both possible ketones results when an unsymmetrically substituted internal alkyne (RC≡CR’) is hydrated. The reaction is therefore most useful when applied to a terminal alkyne (RC≡CH) because only a methyl ketone is formed.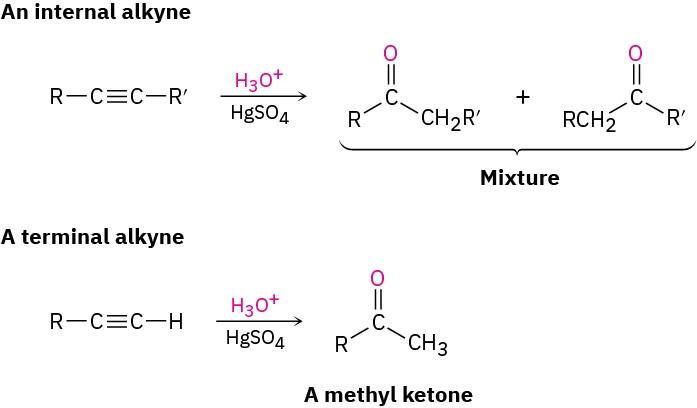 Problem 5.13
Problem 5.13
What products would you obtain by mercury-catalyzed hydration of the following alkynes?
(a) 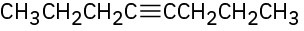
(b) 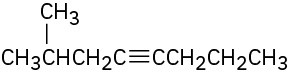
Problem 5.14
What alkynes would you start with to prepare the following ketones?
(a)
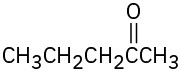
(b)