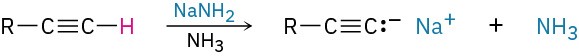Key Terms
- 1,2-addition
- 1,4-addition
- acetylide anion
- alkylation
- alkyne
- allyl group
- anti stereochemistry
- bromonium ion
- carbene, R2C
- chain-growth polymer
- conjugated
- enol
- epoxide
- glycol
- hydrogenated
- hydroxylation
- hyperconjugation
- Lindlar Catalyst
- Markovnikov’s rule
- methylene group
- monomer
- oxidation
- oxirane
- polymer
- reduction
- regiospecific reaction
- syn stereochemistry
- tautomer
- vinyl group
Summary of Reactions Chapter 5
Alkene Reactions
No stereochemistry is implied unless specifically indicated with wedged, solid, and dashed lines.
1. Addition reactions of alkene
- Addition of HCl, HBr, and HI (Section 5.1). Markovnikov regiochemistry occurs, with H adding to the less highly substituted alkene carbon and halogen adding to the more highly substituted carbon.
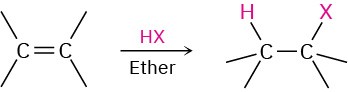
- Addition of halogens Cl2 and Br2 (Section 5.4). Anti addition is observed through a halonium ion intermediate.
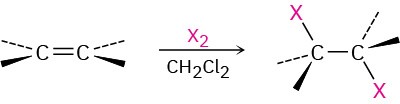
- Catalytic hydrogenation (Section 5.5). Syn addition occurs.
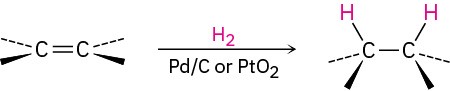
- Epoxidation with a peroxyacid (Section 5.6) Syn addition occurs.
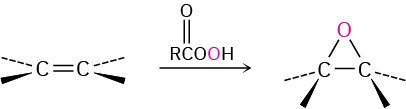
- Hydroxylation with OsO4 (Section 5.6) Syn addition occurs.
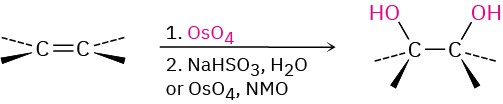
2. Hydroxylation by acid-catalyzed epoxide hydrolysis (Section 5.6) Anti stereochemistry occurs.
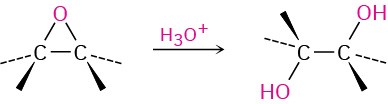
3. Electrophilic addition reactions to conjugated dienes. (Section 5.9)
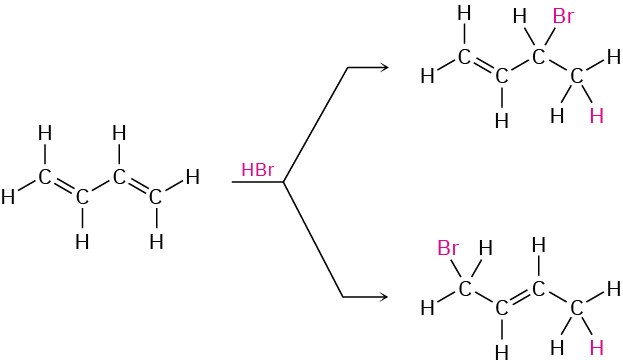
Alkyne Reactions
No stereochemistry is implied unless specifically indicated with wedged, solid, and dashed lines.
1. Preparation of Alkynes
- Alkylation of acetylide anions (Section 5.14)
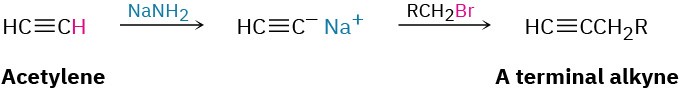
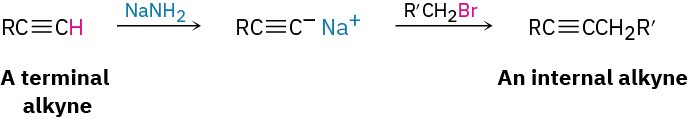
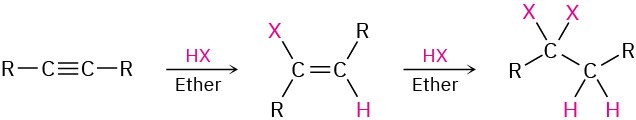
2. Reactions of alkynes
- Addition of HCl and HBr (Section 5.3)
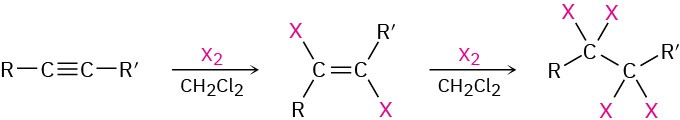
- Addition of Cl2 and Br2 (Section 5.3)
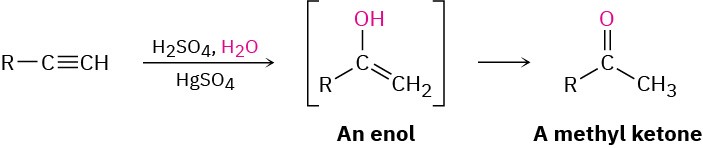
- Mercuric sulfate catalyzed Hydration (Section 5.4)
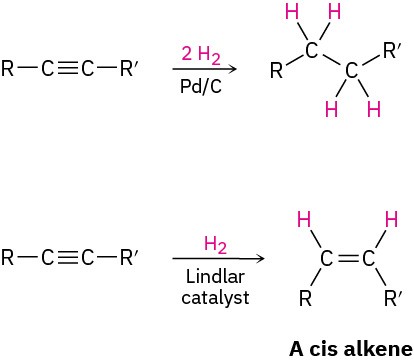
- Catalytic hydrogenation (Section 5.5)
- Conversion into acetylide anions (Section 5.7)