8.2 Naming Aromatic Compounds
Simple aromatic hydrocarbons come from two main sources: coal and petroleum. Coal is an enormously complex mixture consisting primarily of large arrays of benzene-like rings joined together. Thermal breakdown of coal occurs when heated to 1000 °C in the absence of air, and a mixture of volatile products called coal tar boils off. Fractional distillation of coal tar yields benzene, toluene, xylene (dimethylbenzene), naphthalene, and a host of other aromatic compounds (Figure 8.3).
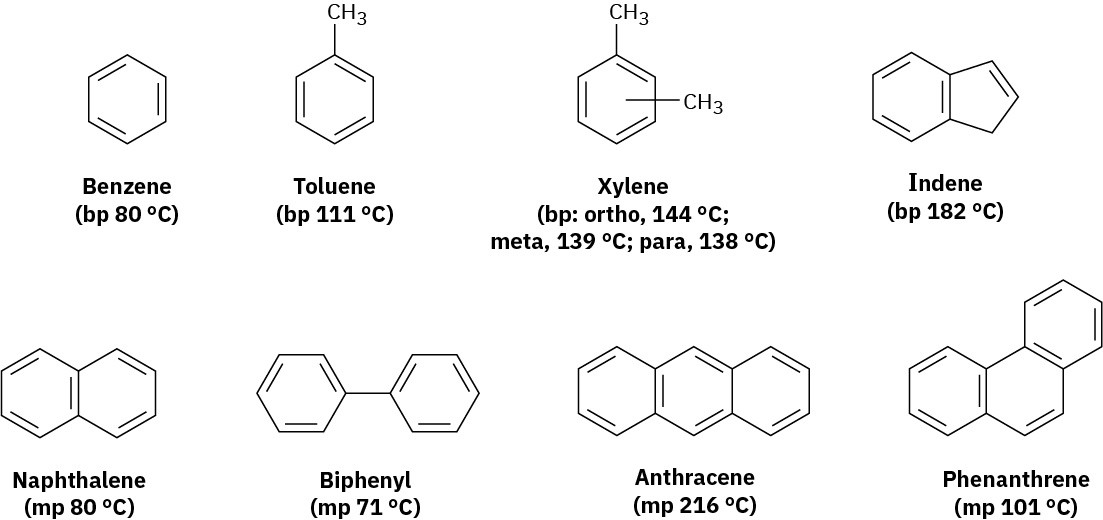 Figure 8.3 Some aromatic hydrocarbons found in coal tar.
Figure 8.3 Some aromatic hydrocarbons found in coal tar.
Unlike coal, petroleum contains few aromatic compounds and consists largely of alkanes (see Chapter 2 Chemistry Matters). During petroleum refining, however, aromatic molecules are formed when alkanes are passed over a catalyst at about 500 °C under high pressure.
Aromatic substances, more than any other class of organic compounds, have acquired a large number of nonsystematic names. IUPAC rules discourage the use of most such names but do allow some of the more widely used ones to be retained (Table 8.1). Thus, methylbenzene is known commonly as toluene; hydroxybenzene as phenol; aminobenzene as aniline; and so on.
Table 8.1 Common Names of Some Aromatic Compounds
|
Structure |
Name |
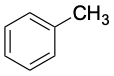 |
Toluene (bp 111 °C) |
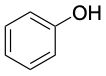 |
Phenol (mp 43 °C) |
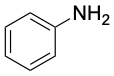 |
Aniline (bp 184 °C) |
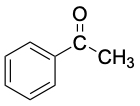 |
Acetophenone (mp 21 °C) |
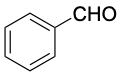 |
Benzaldehyde (bp 178 °C) |
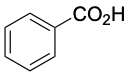 |
Benzoic acid (mp 122 °C) |
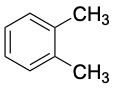 |
ortho-Xylene (bp 144 °C) |
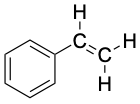 |
Styrene (bp 145 °C) |
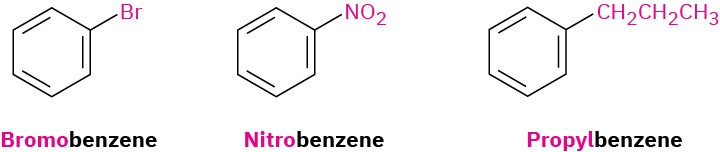
Monosubstituted benzenes are named systematically in the same manner as other hydrocarbons, with –benzene as the parent name. Thus, C6H5Br is bromobenzene, C6H5NO2 is nitrobenzene, and C6H5CH2CH2CH3 is propylbenzene.
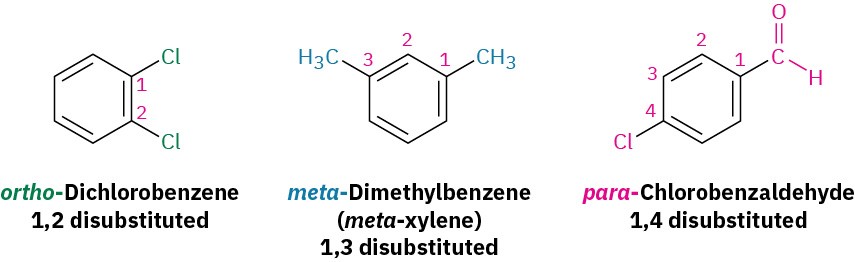
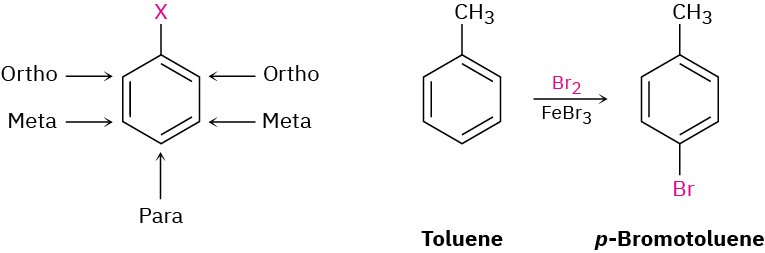
Alkyl-substituted benzenes are sometimes referred to as arenes and are named in different ways depending on the size of the alkyl group. If the alkyl substituent is smaller than the ring (six or fewer carbons), the arene is referred to as an alkyl-substituted benzene. If the alkyl substituent is larger than the ring (seven or more carbons), the compound is referred to as a phenyl-substituted alkane. The name phenyl, pronounced fen-nil and sometimes abbreviated as Ph or Ф (Greek phi), is used for the –C6H5 unit when the benzene ring is considered a substituent. The word is derived from the Greek pheno (“I bear light”), commemorating the discovery of benzene by Michael Faraday in 1825 from the oily residue left by the illuminating gas used in London street lamps. In addition, the name benzyl is used for the C6H5CH2– group.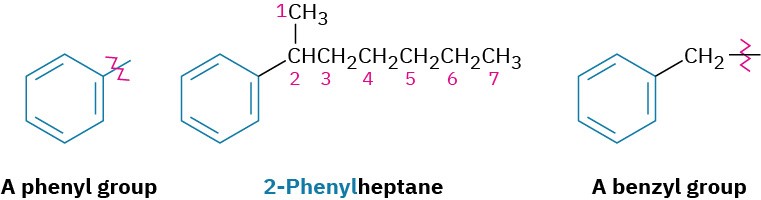 Disubstituted benzenes are named using the prefixes ortho (o), meta (m), or para (p). An ortho-disubstituted benzene has its two substituents in a 1,2 relationship on the ring, a meta-disubstituted benzene has its two substituents in a 1,3 relationship, and a para-disubstituted benzene has its substituents in a 1,4 relationship.
Disubstituted benzenes are named using the prefixes ortho (o), meta (m), or para (p). An ortho-disubstituted benzene has its two substituents in a 1,2 relationship on the ring, a meta-disubstituted benzene has its two substituents in a 1,3 relationship, and a para-disubstituted benzene has its substituents in a 1,4 relationship.
 The ortho, meta, para system of nomenclature is also useful when discussing reactions. For example, we might describe the reaction of bromine with toluene by saying, “Reaction occurs at the para position,” meaning at the position para to the methyl group already present on the ring.
The ortho, meta, para system of nomenclature is also useful when discussing reactions. For example, we might describe the reaction of bromine with toluene by saying, “Reaction occurs at the para position,” meaning at the position para to the methyl group already present on the ring.
 As with cycloalkanes (Section 2.5), benzenes with more than two substituents are named by choosing a point of attachment as carbon 1 and numbering the substituents on the ring so that the second substituent has as low a number as possible. If ambiguity still exists, number so that the third or fourth substituent has as low a number as possible, until a point of difference is found. The substituents are listed alphabetically when writing the name.
As with cycloalkanes (Section 2.5), benzenes with more than two substituents are named by choosing a point of attachment as carbon 1 and numbering the substituents on the ring so that the second substituent has as low a number as possible. If ambiguity still exists, number so that the third or fourth substituent has as low a number as possible, until a point of difference is found. The substituents are listed alphabetically when writing the name.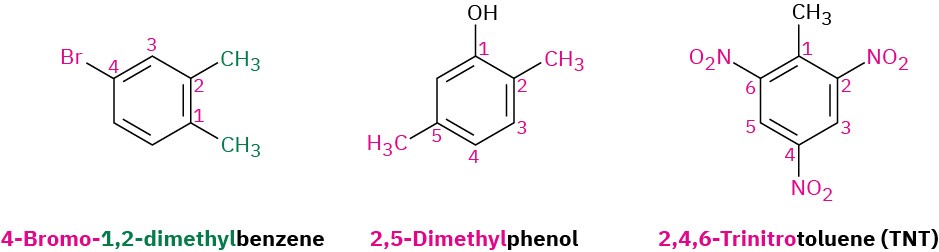 Note in the second and third examples shown that –phenol and –toluene are used as the parent names rather than –benzene. Any of the monosubstituted aromatic compounds shown in Table 8.1 can serve as a parent name, with the principal substituent (–OH in phenol or –CH3 in toluene) attached to C1 on the ring.
Note in the second and third examples shown that –phenol and –toluene are used as the parent names rather than –benzene. Any of the monosubstituted aromatic compounds shown in Table 8.1 can serve as a parent name, with the principal substituent (–OH in phenol or –CH3 in toluene) attached to C1 on the ring.
Problem 8.2
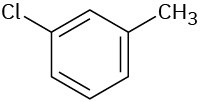
Tell whether the following compounds are ortho-, meta-, or para-disubstituted:
(a)
(b)
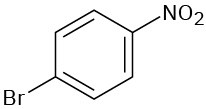
(c)
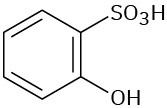
Problem 8.3
Give IUPAC names for the following compounds:
(a)
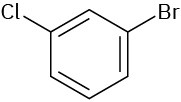
(b)
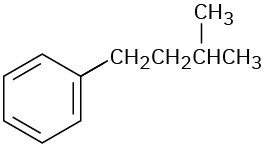
(c)
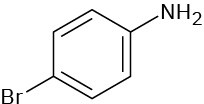
(d)
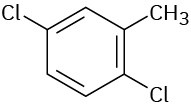
(e)
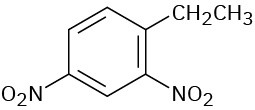
(f)
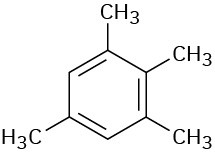
Problem 8.4
Draw structures corresponding to the following IUPAC names:
(a) p-Bromochlorobenzene
(b) p-Bromotoluene
(c) m-Chloroaniline
(d) 1-Chloro-3,5-dimethylbenzene

