11.5 Nucleophilic Acyl Substitution Reactions
The addition of a nucleophile to a polar C═O bond is the key step in three of the four major carbonyl-group reactions. We saw in the chapter on Aldehydes and Ketones: Nucleophilic Addition Reactions that when a nucleophile adds to an aldehyde or ketone, the initially formed tetrahedral intermediate can be protonated to yield an alcohol. When a nucleophile adds to a carboxylic acid derivative, however, a different reaction path is taken. The initially formed tetrahedral intermediate eliminates one of the two substituents originally bonded to the carbonyl carbon, leading to a net nucleophilic acyl substitution reaction (Figure 11.3).
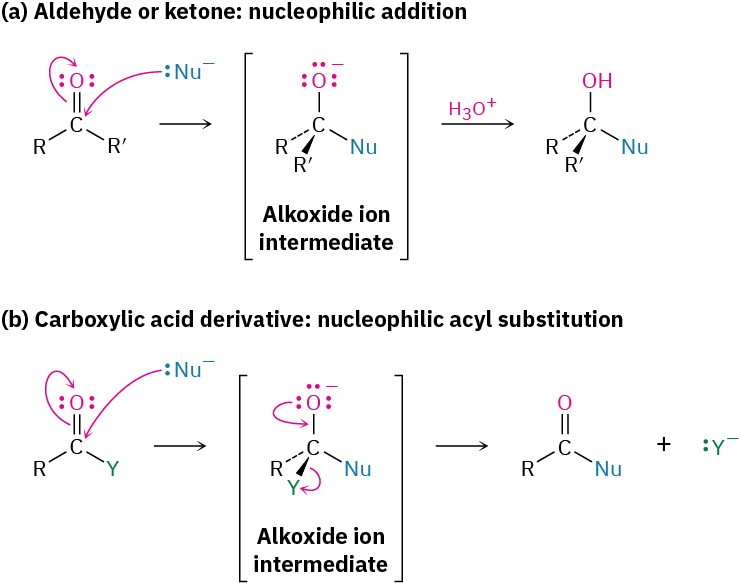 Figure 11.3 The general mechanisms of nucleophilic addition and nucleophilic acyl substitution reactions. Both reactions begin with addition of a nucleophile to a polar C═O bond to give a tetrahedral, alkoxide ion intermediate. (a) The intermediate formed from an aldehyde or ketone is protonated to give an alcohol, but (b) the intermediate formed from a carboxylic acid derivative expels a leaving group to give a new carbonyl compound.
Figure 11.3 The general mechanisms of nucleophilic addition and nucleophilic acyl substitution reactions. Both reactions begin with addition of a nucleophile to a polar C═O bond to give a tetrahedral, alkoxide ion intermediate. (a) The intermediate formed from an aldehyde or ketone is protonated to give an alcohol, but (b) the intermediate formed from a carboxylic acid derivative expels a leaving group to give a new carbonyl compound.
The difference in behavior between aldehydes/ketones and carboxylic acid derivatives is a consequence of structure. Carboxylic acid derivatives have an acyl carbon bonded to a group –Y that can act as a leaving group, often as a stable anion. As soon as the tetrahedral intermediate is formed, the leaving group is expelled to generate a new carbonyl compound. Aldehydes and ketones, however, have no such leaving group, however, and therefore don’t undergo substitution.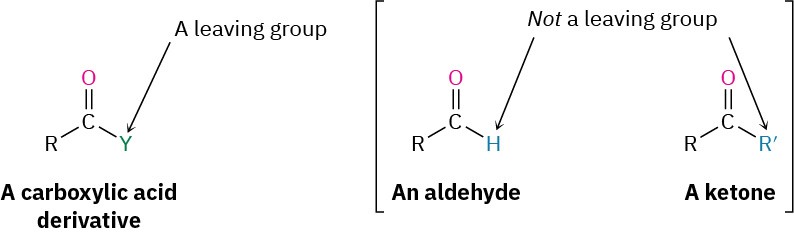
The net effect of the addition/elimination sequence is a substitution of the nucleophile for the –Y group that was originally bonded to the acyl carbon. Thus, the overall reaction is superficially similar to the kind of nucleophilic substitution that occurs during an SN2 reaction (Section 7.3), but the mechanisms of the two reactions are completely different. An SN2 reaction occurs in a single step by backside displacement of the leaving group, while a nucleophilic acyl substitution takes place in two steps and involves a tetrahedral intermediate.
Problem 11.10
Show the mechanism of the following nucleophilic acyl substitution reaction, using curved arrows to indicate the electron flow in each step:
Relative Reactivity of Carboxylic Acid Derivatives
Both the initial addition step and the subsequent elimination step can affect the overall rate of a nucleophilic acyl substitution reaction, but the addition is usually the rate-limiting step. Thus, any factor that makes the carbonyl group more reactive toward nucleophiles favors the substitution process.
Steric and electronic factors are both important in determining reactivity. Sterically, we find within a series of similar acid derivatives that unhindered, accessible carbonyl groups react with nucleophiles more readily than do sterically hindered carbonyl groups. The reactivity order is
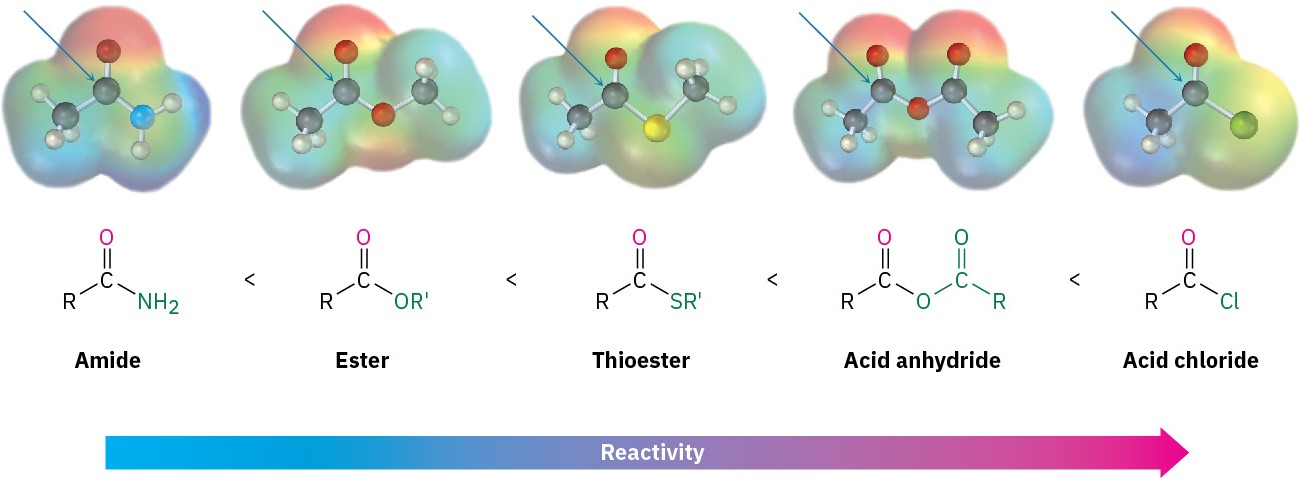
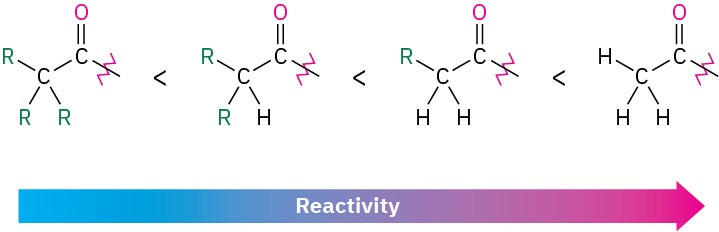 Electronically, we find that strongly polarized acyl compounds react more readily than less polar ones. Thus, acid chlorides are the most reactive because the electronegative chlorine atom withdraws electrons from the carbonyl carbon, whereas amides are the least reactive. Although subtle, electrostatic potential maps of various carboxylic acid derivatives indicate these differences by the relative blueness on the C═O carbons. Acyl phosphates are hard to place on this scale because they are not often used in the laboratory, but in biological systems they appear to be somewhat more reactive than thioesters.
Electronically, we find that strongly polarized acyl compounds react more readily than less polar ones. Thus, acid chlorides are the most reactive because the electronegative chlorine atom withdraws electrons from the carbonyl carbon, whereas amides are the least reactive. Although subtle, electrostatic potential maps of various carboxylic acid derivatives indicate these differences by the relative blueness on the C═O carbons. Acyl phosphates are hard to place on this scale because they are not often used in the laboratory, but in biological systems they appear to be somewhat more reactive than thioesters.
 As a consequence of these reactivity differences, it’s usually possible to convert a more reactive acid derivative into a less reactive one. Acid chlorides, for instance, can be directly converted into anhydrides, thioesters, esters, and amides, but amides can’t be directly converted into esters, thioesters, anhydrides, or acid chlorides. Remembering the reactivity order is therefore a way to keep track of a large number of reactions (Figure 11.4). Another consequence, as noted previously, is that only acyl phosphates, thioesters, esters, and amides are commonly found in nature. Acid halides and acid anhydrides react so rapidly with water that they can’t exist for long in living organisms.
As a consequence of these reactivity differences, it’s usually possible to convert a more reactive acid derivative into a less reactive one. Acid chlorides, for instance, can be directly converted into anhydrides, thioesters, esters, and amides, but amides can’t be directly converted into esters, thioesters, anhydrides, or acid chlorides. Remembering the reactivity order is therefore a way to keep track of a large number of reactions (Figure 11.4). Another consequence, as noted previously, is that only acyl phosphates, thioesters, esters, and amides are commonly found in nature. Acid halides and acid anhydrides react so rapidly with water that they can’t exist for long in living organisms.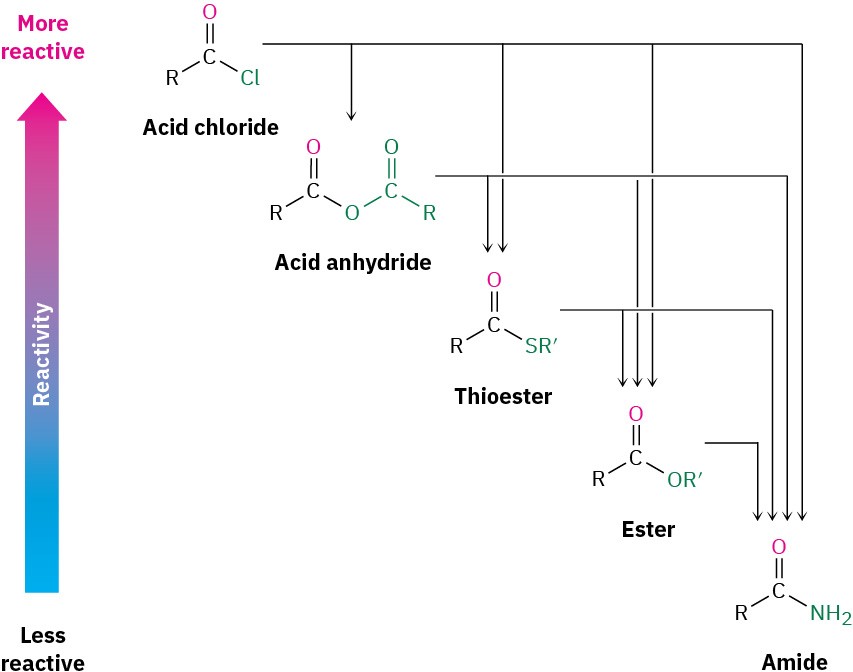 Figure 11.4 Interconversions of carboxylic acid derivatives. A more reactive acid derivative can be converted into a less reactive one, but not vice versa.
Figure 11.4 Interconversions of carboxylic acid derivatives. A more reactive acid derivative can be converted into a less reactive one, but not vice versa.
In studying the chemistry of carboxylic acid derivatives in the next few sections, we’ll be concerned largely with the reactions of just a few nucleophiles and will see that the same kinds of reactions tend to reoccur (Figure 11.5).
|
Hydrolysis |
Reaction with water to yield a carboxylic acid |
|
Alcoholysis |
Reaction with an alcohol to yield an ester |
|
Aminolysis |
Reaction with ammonia or an amine to yield an amide |
|
Reduction |
Reaction with a hydride reducing agent to yield an aldehyde or an alcohol |
|
Grignard reaction |
Reaction with an organometallic reagent to yield a ketone or an alcohol |
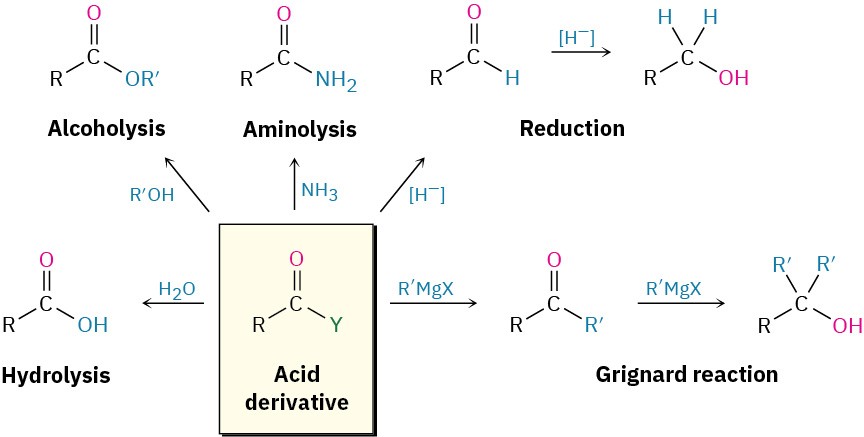
Figure 11.5 Some general reactions of carboxylic acid derivatives.
Worked Example 11.1: Predicting the Product of a Nucleophilic Acyl Substitution Reaction
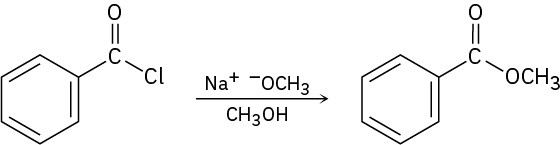
Predict the product of the following nucleophilic acyl substitution reaction of benzoyl chloride with 2-propanol:
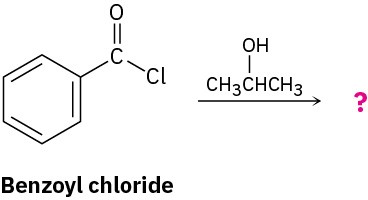
Strategy
A nucleophilic acyl substitution reaction involves the substitution of a nucleophile for a leaving group in a carboxylic acid derivative. Identify the leaving group (Cl– in the case of an acid chloride) and the nucleophile (an alcohol in this case), and replace one by the other. The product is isopropyl benzoate.
Solution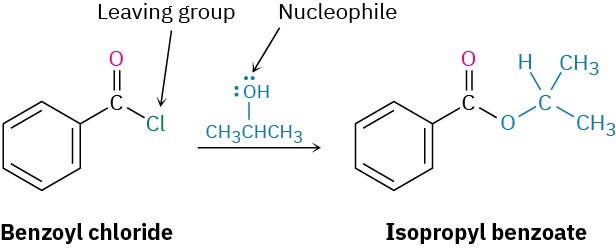 Problem 11.11
Problem 11.11
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acyl substitution:
(a)
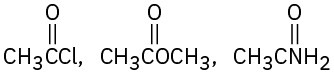
(b)
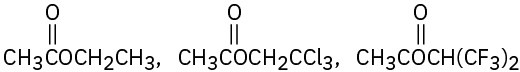
Problem 11.12
Predict the products of the following nucleophilic acyl substitution reactions:
(a)
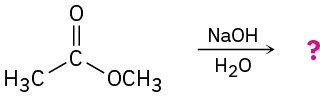
(b)
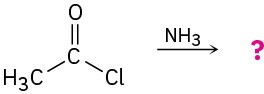
(c)
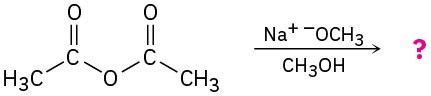
(d)
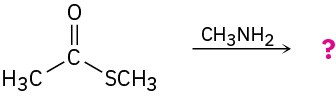
Problem 11.13
The following structure represents a tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting acid derivative, and the ultimate product.