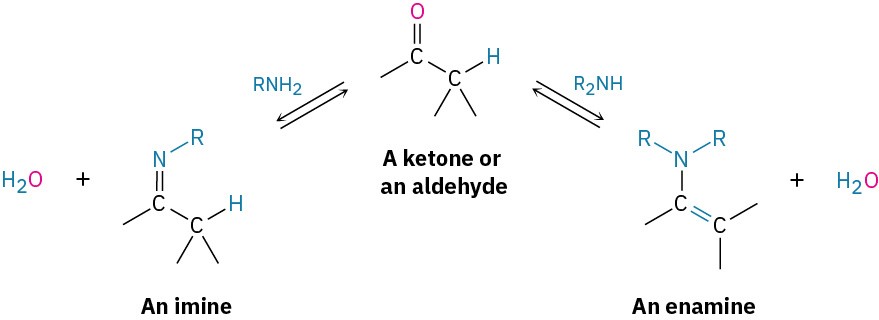
10.8 Nucleophilic Addition of Amines: Imine and Enamine Formation
Primary amines, RNH2, add to aldehydes and ketones to yield imines, R2C═NR. Secondary amines, R2NH, add similarly to yield enamines, R2N–CR═CR2 (ene + amine = unsaturated amine).
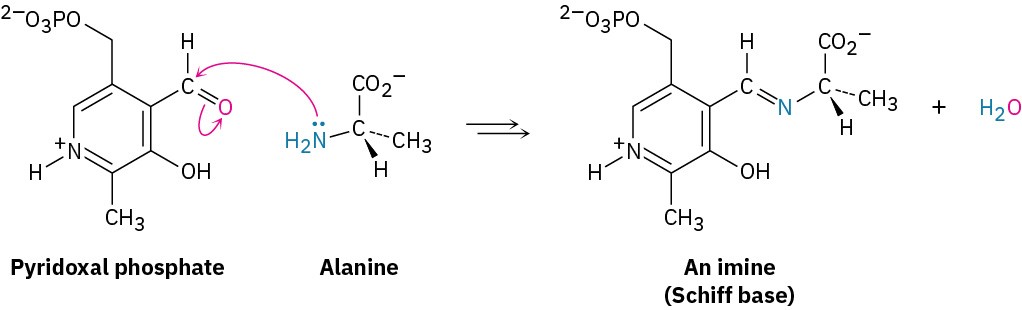
Imines are particularly common as intermediates in biological pathways, where they are often called Schiff bases. The amino acid alanine, for instance, is metabolized in the body by reaction with the aldehyde pyridoxal phosphate (PLP), a derivative of vitamin B6, to yield a Schiff base that is further degraded.
 Imine formation and enamine formation seem different because one leads to a product with a C═N bond and the other leads to a product with a C═C bond. Actually, though, the reactions are quite similar. Both are typical examples of nucleophilic addition reactions in which water is eliminated from the initially formed tetrahedral intermediate and a new C═Nu double bond is formed.
Imine formation and enamine formation seem different because one leads to a product with a C═N bond and the other leads to a product with a C═C bond. Actually, though, the reactions are quite similar. Both are typical examples of nucleophilic addition reactions in which water is eliminated from the initially formed tetrahedral intermediate and a new C═Nu double bond is formed.
Imines are formed in a reversible, acid-catalyzed process (Figure 10.7) that begins with nucleophilic addition of the primary amine to the carbonyl group, followed by transfer of a proton from nitrogen to oxygen to yield a neutral amino alcohol, or carbinolamine.
Protonation of the carbinolamine oxygen by an acid catalyst then converts the –OH into a better leaving group (–OH2+), and E1-like loss of water produces an iminium ion. Loss of a proton from nitrogen gives the final product and regenerates the acid catalyst.
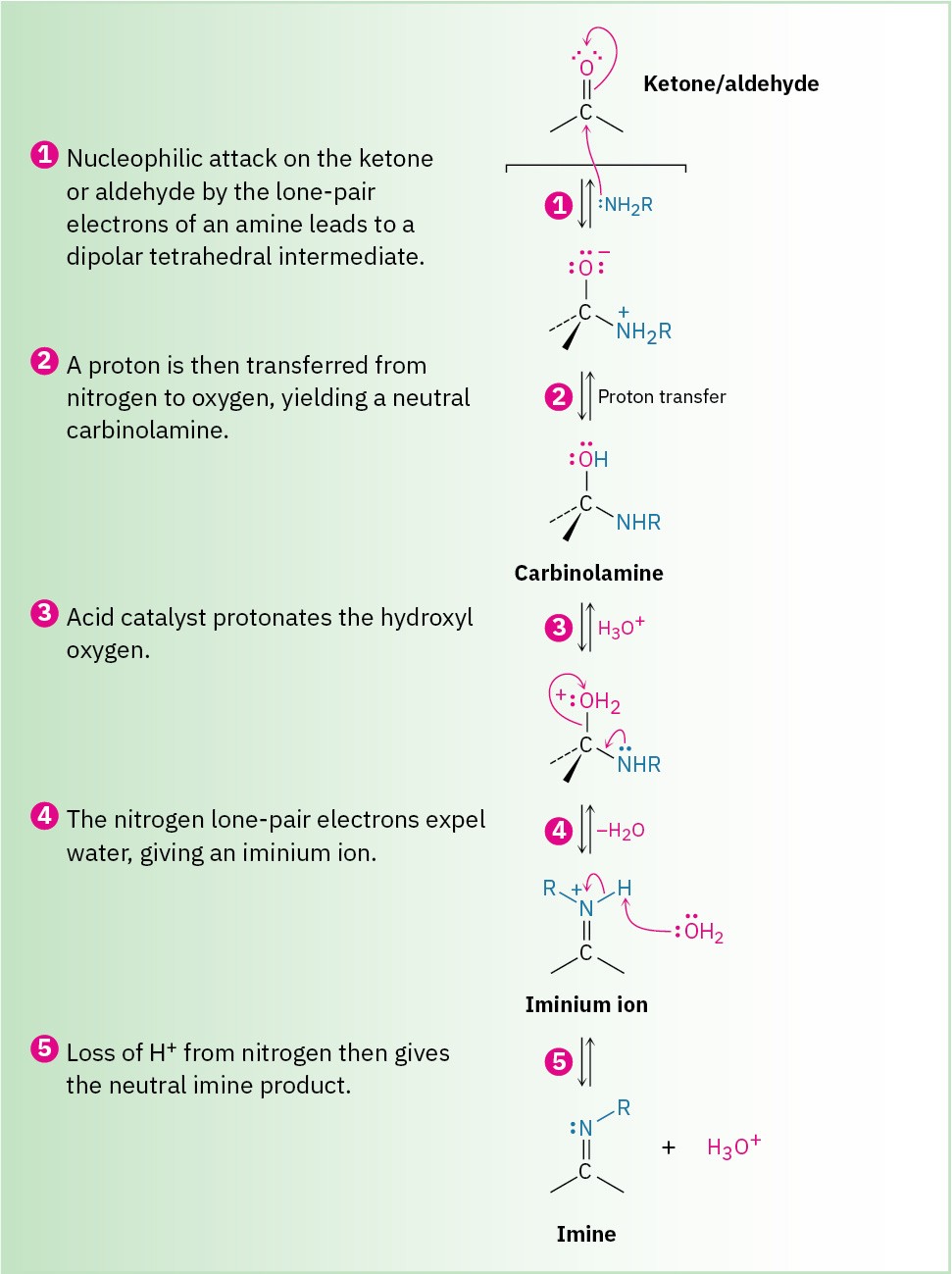
Figure 10.7 MECHANISM: Mechanism of imine formation by reaction of an aldehyde or ketone with a primary amine. The key step is the initial nucleophilic addition to yield a carbinolamine intermediate, which then loses water to give the imine.
Imine formation with such reagents as hydroxylamine and 2,4-dinitrophenylhydrazine is sometimes useful because the products of these reactions—oximes and 2,4- dinitrophenylhydrazones (2,4-DNPs), respectively—are often crystalline and easy to handle. Such crystalline derivatives are occasionally prepared as a means of purifying and characterizing liquid ketones or aldehydes.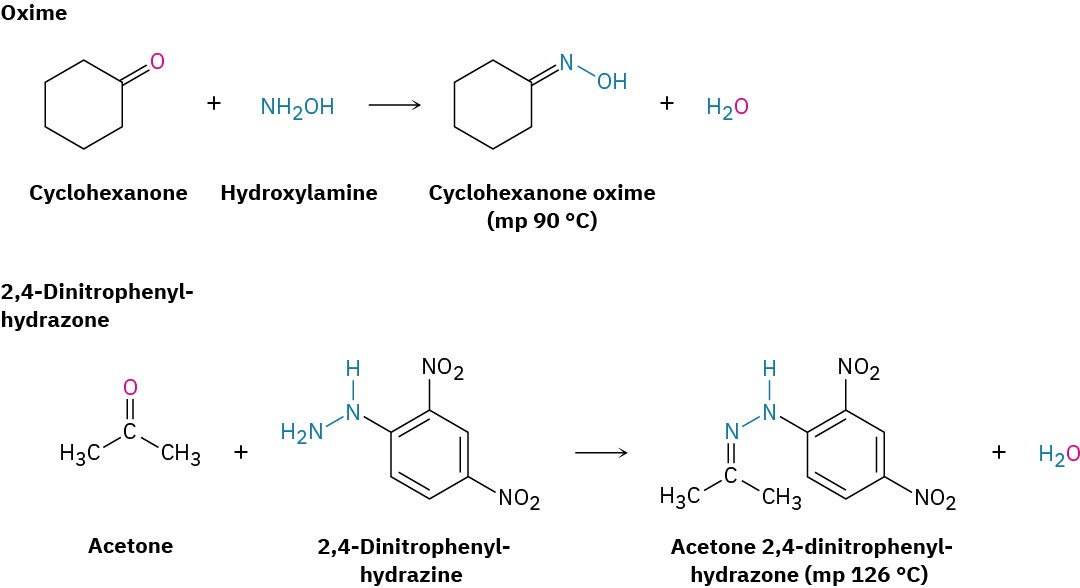 Reaction of an aldehyde or ketone with a secondary amine, R2NH, rather than a primary amine yields an enamine.
Reaction of an aldehyde or ketone with a secondary amine, R2NH, rather than a primary amine yields an enamine.
Imine and enamine formation are slow at both high pH and low pH but reach a maximum rate at a weakly acidic pH around 4 to 5.
Problem 10.9
Show the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2.

