10.6 Nucleophilic Addition of HCN: Cyanohydrin Formation
Aldehydes and unhindered ketones undergo a nucleophilic addition reaction with HCN to yield cyanohydrins, RCH(OH)C≡N. Studies carried out in the early 1900s by Arthur Lapworth showed that cyanohydrin formation is reversible and base-catalyzed. Reaction occurs slowly when pure HCN is used but rapidly when a small amount of base is added to generate the nucleophilic cyanide ion, CN–. Addition of CN– takes place by a typical nucleophilic addition pathway, yielding a tetrahedral intermediate that is protonated by HCN to give cyanohydrin product plus regenerated CN–.
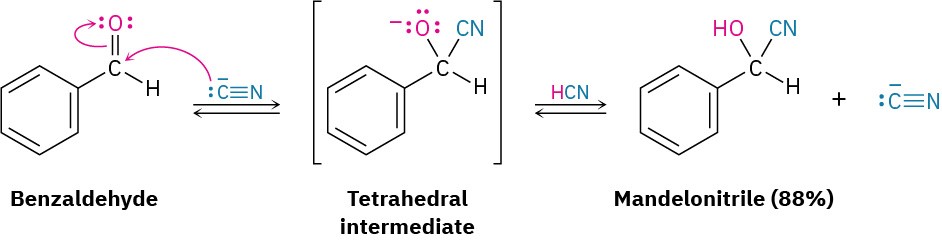 Cyanohydrin formation is somewhat unusual because it is one of the few examples of the addition of a protic acid (H–Y) to a carbonyl group. As noted in the previous section, protic acids such as H2O, HBr, HCl, and H2SO4 don’t normally yield carbonyl addition products because the equilibrium constants are unfavorable. With HCN, however, equilibrium favors the cyanohydrin adduct.
Cyanohydrin formation is somewhat unusual because it is one of the few examples of the addition of a protic acid (H–Y) to a carbonyl group. As noted in the previous section, protic acids such as H2O, HBr, HCl, and H2SO4 don’t normally yield carbonyl addition products because the equilibrium constants are unfavorable. With HCN, however, equilibrium favors the cyanohydrin adduct.
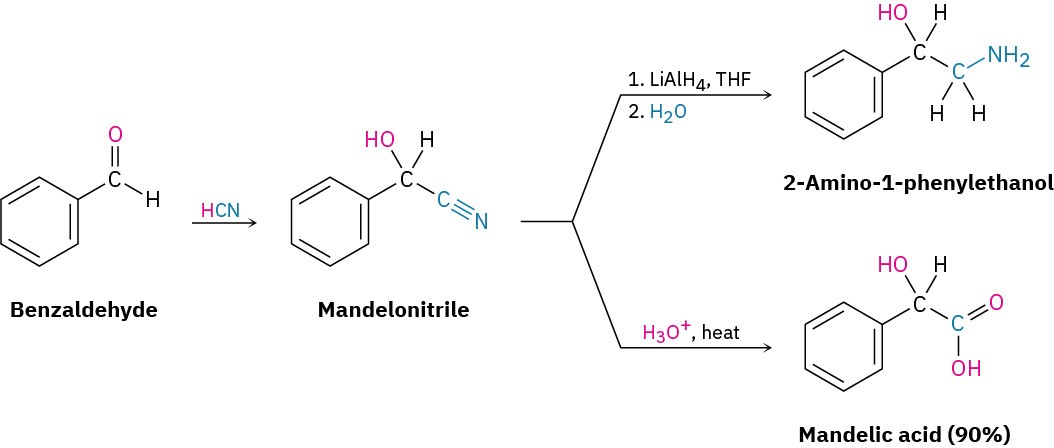
Cyanohydrin formation is useful because of the further chemistry that can be carried out on the product. For example, a nitrile (R–C≡N) can be reduced with LiAlH4 to yield a primary amine (RCH2NH2) and can be hydrolyzed by hot aqueous acid to yield a carboxylic acid.
Thus, cyanohydrin formation provides a method for transforming an aldehyde or ketone into a different functional group.