8.4 Other Aromatic Substitutions
There are many other kinds of electrophilic aromatic substitutions besides bromination, and all occur by the same general mechanism. Let’s look at some of these other reactions briefly.
Aromatic Halogenation
Chlorine and iodine can be introduced into aromatic rings by electrophilic substitution reactions just as bromine can, but fluorine is too reactive and only poor yields of monofluoroaromatic products are obtained by direct fluorination. Aromatic rings react with Cl2 in the presence of FeCl3 catalyst to yield chlorobenzenes, just as they react with Br2 and FeBr3. This kind of reaction is used in the synthesis of numerous pharmaceutical agents, including the antiallergy medication loratadine, marketed as Claritin.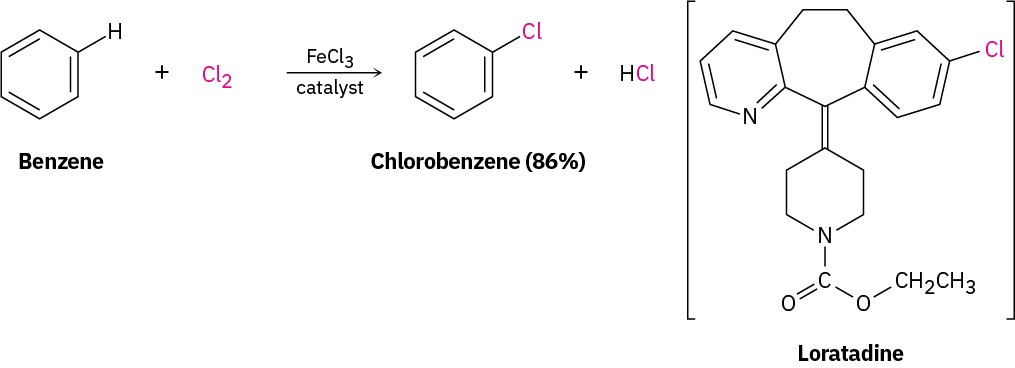 Electrophilic aromatic halogenations also occur in the biosynthesis of many naturally occurring molecules, particularly those produced by marine organisms. In humans, the best-known example occurs in the thyroid gland during the biosynthesis of thyroxine, a hormone involved in regulating growth and metabolism. The amino acid tyrosine is first iodinated by thyroid peroxidase, and two of the iodinated tyrosine molecules then couple. The electrophilic iodinating agent is an I+ species, perhaps hypoiodous acid (HIO), that is formed from iodide ion by oxidation with H2O2.
Electrophilic aromatic halogenations also occur in the biosynthesis of many naturally occurring molecules, particularly those produced by marine organisms. In humans, the best-known example occurs in the thyroid gland during the biosynthesis of thyroxine, a hormone involved in regulating growth and metabolism. The amino acid tyrosine is first iodinated by thyroid peroxidase, and two of the iodinated tyrosine molecules then couple. The electrophilic iodinating agent is an I+ species, perhaps hypoiodous acid (HIO), that is formed from iodide ion by oxidation with H2O2.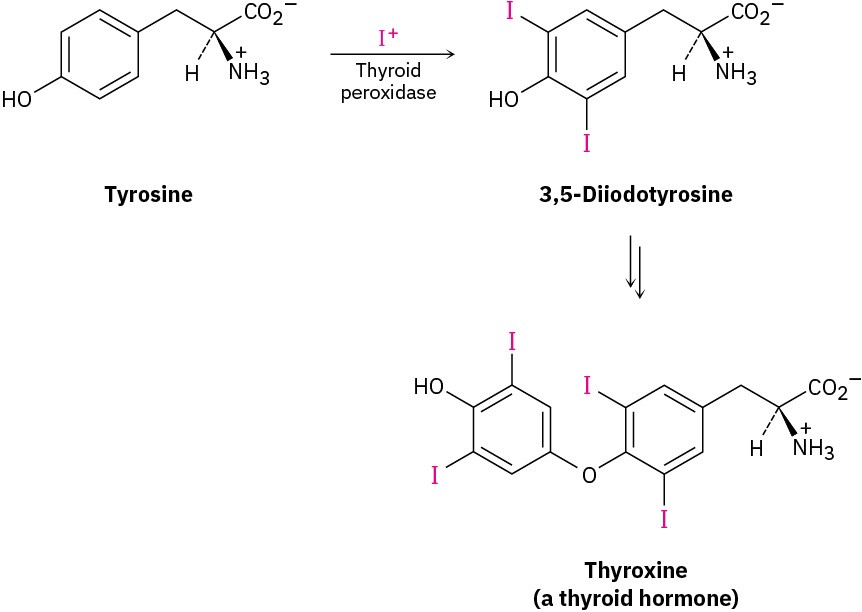
Aromatic Nitration
Aromatic rings are nitrated by reaction with a mixture of concentrated nitric and sulfuric acids. The electrophile is the nitronium ion, NO2+, which is formed from HNO3 by protonation and loss of water. The nitronium ion reacts with benzene to yield a carbocation intermediate, and loss of H+ from this intermediate gives the neutral substitution product, nitrobenzene (Figure 8.7).
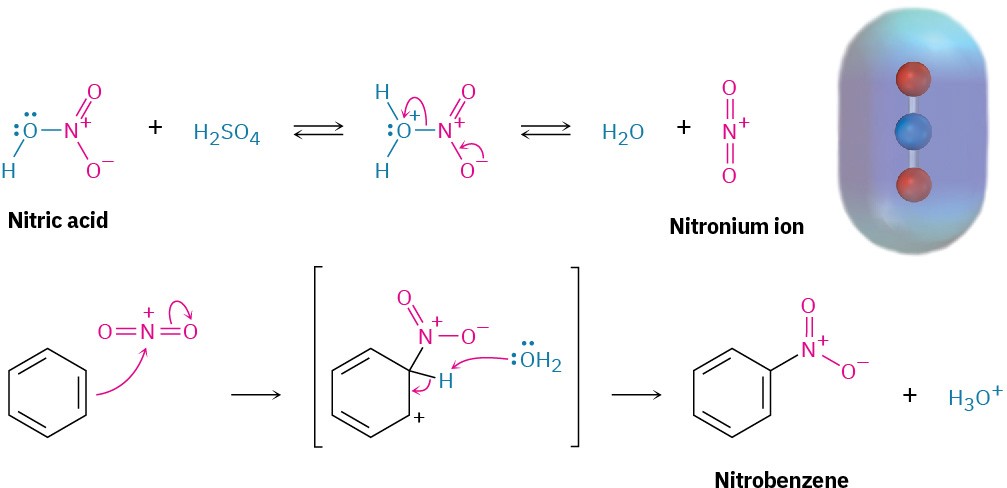 Figure 8.7 The mechanism for electrophilic nitration of an aromatic ring. An electrostatic potential map of the reactive electrophile NO2+ shows that the nitrogen atom is most positive.
Figure 8.7 The mechanism for electrophilic nitration of an aromatic ring. An electrostatic potential map of the reactive electrophile NO2+ shows that the nitrogen atom is most positive.
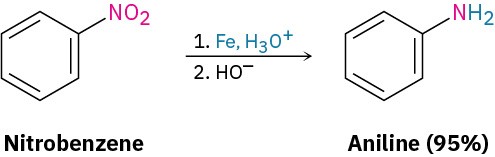
Electrophilic nitration of an aromatic ring does not occur in nature but is particularly important in the laboratory because the nitro-substituted product can be reduced by reagents such as iron, tin, or SnCl2 to yield the corresponding arylamine, ArNH2.
Attachment of an amino group (–NH2) to an aromatic ring by the two-step nitration/reduction sequence is a key part of the industrial synthesis of many dyes and pharmaceutical agents. Recall that we’ve discussed this reduction and other reactions of aromatic nitrogen compounds in Chapter 7.
Aromatic Sulfonation
Aromatic rings can be sulfonated by reaction with so-called fuming sulfuric acid, a mixture of H2SO4 and SO3. The reactive electrophile is either HSO3+ or neutral SO3, depending on reaction conditions, and substitution occurs by the same two-step mechanism seen previously for bromination and nitration (Figure 8.8). 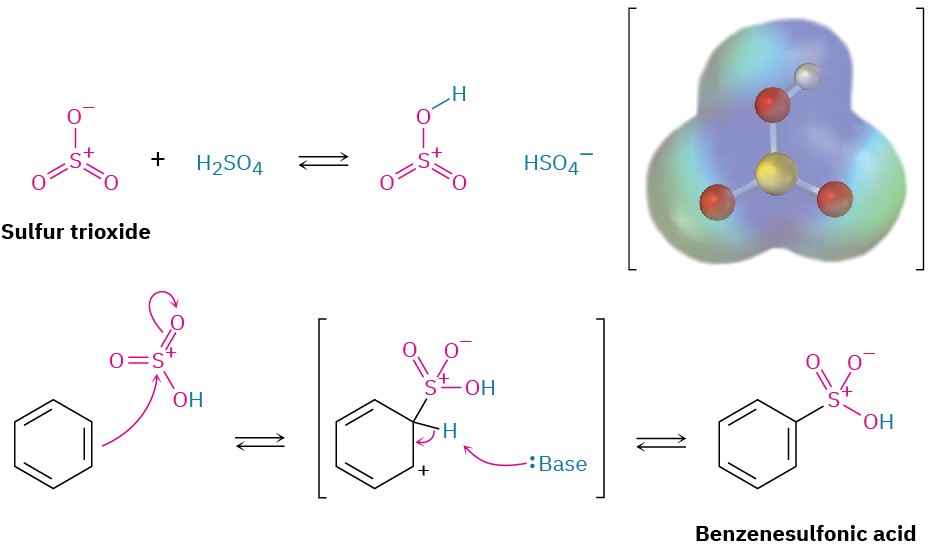 Figure 8.8 The mechanism for electrophilic sulfonation of an aromatic ring. An electrostatic potential map of the reactive electrophile HOSO2+ shows that sulfur and hydrogen are the most positive atoms.
Figure 8.8 The mechanism for electrophilic sulfonation of an aromatic ring. An electrostatic potential map of the reactive electrophile HOSO2+ shows that sulfur and hydrogen are the most positive atoms.
Aromatic sulfonation does not occur naturally but is widely used in the preparation of dyes and pharmaceutical agents. For example, the sulfa drugs, such as sulfanilamide, were among the first clinically useful antibiotics. Although largely replaced today by more effective agents, sulfa drugs are still used in the treatment of meningitis and urinary tract infections. These drugs are prepared commercially by a process that involves aromatic sulfonation as its key step.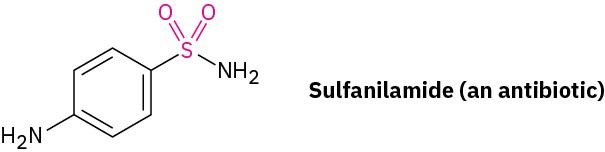
Problem 8.6
How many products might be formed on chlorination of o-xylene (o-dimethylbenzene), m– xylene, and p-xylene?

