10.3 Oxidation of Aldehydes and Ketones
Aldehydes are easily oxidized to yield carboxylic acids, but ketones are generally inert toward oxidation. The difference is a consequence of structure: aldehydes have a –CHO proton that can be abstracted during oxidation, but ketones do not.
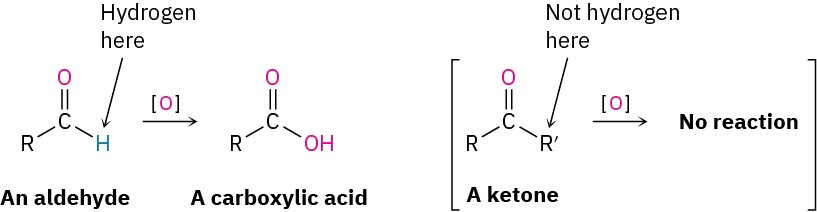 Many oxidizing agents, including alkaline KMnO4 and hot HNO3, convert aldehydes into carboxylic acids. The oxidation occurs rapidly at room temperature and generally works well.
Many oxidizing agents, including alkaline KMnO4 and hot HNO3, convert aldehydes into carboxylic acids. The oxidation occurs rapidly at room temperature and generally works well.
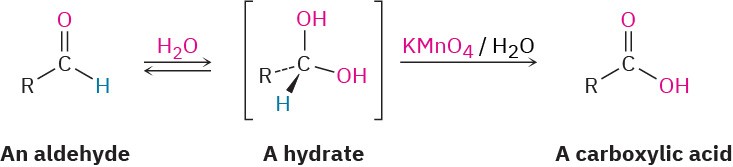
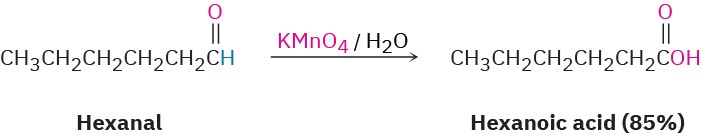 Aldehyde oxidations occur through intermediate 1,1-diols, or hydrates, which are formed by a reversible nucleophilic addition of water to the carbonyl group. Even though it’s formed to only a small extent at equilibrium, the hydrate reacts like any typical primary or secondary alcohol and is oxidized to a carbonyl compound (Section 9.8).
Aldehyde oxidations occur through intermediate 1,1-diols, or hydrates, which are formed by a reversible nucleophilic addition of water to the carbonyl group. Even though it’s formed to only a small extent at equilibrium, the hydrate reacts like any typical primary or secondary alcohol and is oxidized to a carbonyl compound (Section 9.8).
 Ketones are inert to most oxidizing agents.
Ketones are inert to most oxidizing agents.

