1.13 Polar Covalent Bonds and Dipole Moments
Just as individual bonds are often polar, molecules as a whole are often polar as well. Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule. As a practical matter, strongly polar substances are often soluble in polar solvents like water, whereas less polar substances are insoluble in water.
Net polarity is measured by a quantity called the dipole moment. Dipole moments are represented by the lowercase Greek letter mu, (μ) and are measured in units of debyes (D). The dipole moment can be thought of in the following way: assume that there is a center of mass of all positive charges (nuclei) in a molecule and a center of mass of all negative charges (electrons). If these two centers don’t coincide, then the molecule has a net polarity.
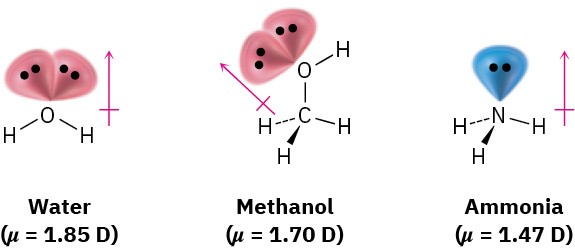
Dipole moments for some common substances are given in Table 1.4. Of the compounds shown in the table, sodium chloride has the largest dipole moment (9.00 D) because it is ionic. Even small molecules like water (μ = 1.85 D), methanol (CH3OH; μ = 1.70 D), and ammonia (μ = 1.47 D), have substantial dipole moments, however, both because they contain strongly electronegative atoms (oxygen and nitrogen) and because all three molecules have lone-pair electrons. The lone-pair electrons on oxygen and nitrogen stick out into space away from the positively charged nuclei, giving rise to a considerable charge separation and making a large contribution to the dipole moment.
Table 1.4 Dipole Moments of Some Compounds
|
Compound |
Dipole moment (D) |
Compound |
Dipole moment (D) |
|
NaCl |
9.00 |
NH3 |
1.47 |
|
CH2O |
2.33 |
CH3NH2 |
1.31 |
|
CH3Cl |
1.87 |
CO2 |
0 |
|
H2O |
1.85 |
CH4 |
0 |
|
CH3OH |
1.70 |
CH3CH3 |
0 |
|
CH3CO2H |
1.70 |
 |
0 |
|
CH3SH |
1.52 |
|
|
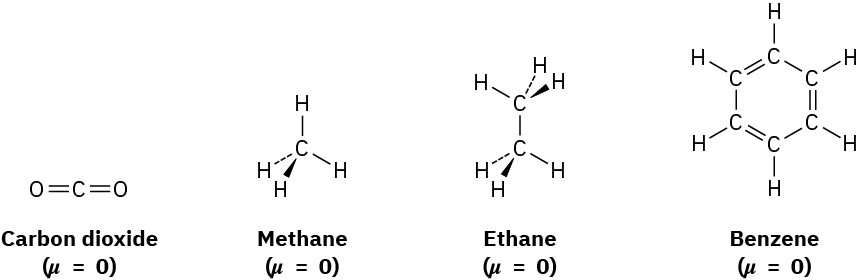
In contrast with water, methanol, and ammonia, molecules such as carbon dioxide, methane, ethane, and benzene have zero dipole moments. Because of the symmetrical structures of these molecules, the individual bond polarities and lone-pair contributions exactly cancel.
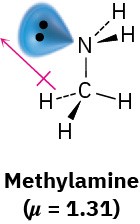
 Worked Example 1.5: Predicting the Direction of a Dipole Moment
Worked Example 1.5: Predicting the Direction of a Dipole Moment
Make a three-dimensional drawing of methylamine, CH3NH2, and show the direction of its dipole moment (μ = 1.31).
Strategy
Look for any lone-pair electrons, and identify any atom with an electronegativity substantially different from that of carbon. (Usually, this means O, N, F, Cl, or Br.) Electron density will be displaced in the general direction of the electronegative atoms and the lone pairs.
Solution
Methylamine contains an electronegative nitrogen atom with a lone pair of electrons. The dipole moment thus points generally from –CH3 toward the lone pair.