6.3 Preparing Alkyl Halides from Alkenes
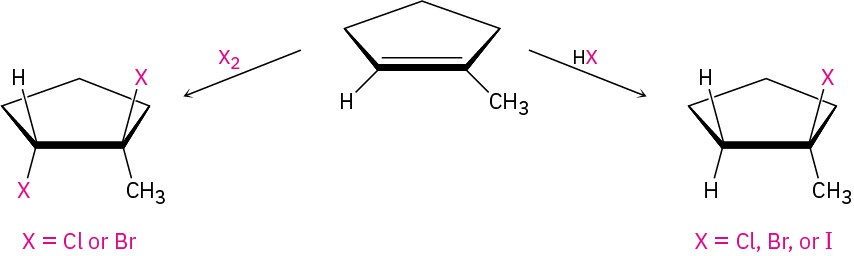
We’ve already seen several methods for preparing alkyl halides from alkenes, including the reactions of HX and X2 with alkenes in electrophilic addition reactions (Section 5.1 and Section 5.4). The hydrogen halides HCl, HBr, and HI react with alkenes by a polar mechanism to give the product of Markovnikov addition. Bromine and chlorine undergo anti addition through halonium ion intermediates to give 1,2-dihalogenated products.
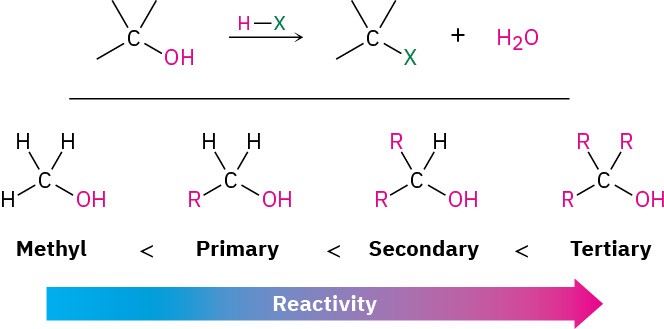
 The most generally useful method for preparing alkyl halides is to make them from alcohols, which themselves can be obtained from carbonyl compounds as we’ll see in Section 9.5 and Section 9.6. Because of the importance of this process, many different methods have been developed to transform alcohols into alkyl halides. The simplest method is to treat the alcohol with HCl, HBr, or HI. For reasons that will be discussed in Section 7.5, this reaction works best with tertiary alcohols, R3COH. Primary and secondary alcohols react much more slowly and at higher temperatures.
The most generally useful method for preparing alkyl halides is to make them from alcohols, which themselves can be obtained from carbonyl compounds as we’ll see in Section 9.5 and Section 9.6. Because of the importance of this process, many different methods have been developed to transform alcohols into alkyl halides. The simplest method is to treat the alcohol with HCl, HBr, or HI. For reasons that will be discussed in Section 7.5, this reaction works best with tertiary alcohols, R3COH. Primary and secondary alcohols react much more slowly and at higher temperatures.
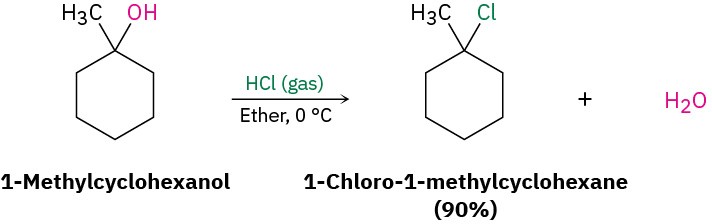
 The reaction of HX with a tertiary alcohol is so rapid that it’s often carried out simply by bubbling pure HCl or HBr gas into a cold ether solution of the alcohol. 1- Methylcyclohexanol, for example, is converted into 1-chloro-1-methylcyclohexane by treatment with HCl.
The reaction of HX with a tertiary alcohol is so rapid that it’s often carried out simply by bubbling pure HCl or HBr gas into a cold ether solution of the alcohol. 1- Methylcyclohexanol, for example, is converted into 1-chloro-1-methylcyclohexane by treatment with HCl.
 Primary and secondary alcohols are best converted into alkyl halides by treatment with either thionyl chloride (SOCl2) or phosphorus tribromide (PBr3). These reactions, which normally take place readily under mild conditions, are less acidic and less likely to cause acid-catalyzed rearrangements than the HX method.
Primary and secondary alcohols are best converted into alkyl halides by treatment with either thionyl chloride (SOCl2) or phosphorus tribromide (PBr3). These reactions, which normally take place readily under mild conditions, are less acidic and less likely to cause acid-catalyzed rearrangements than the HX method.
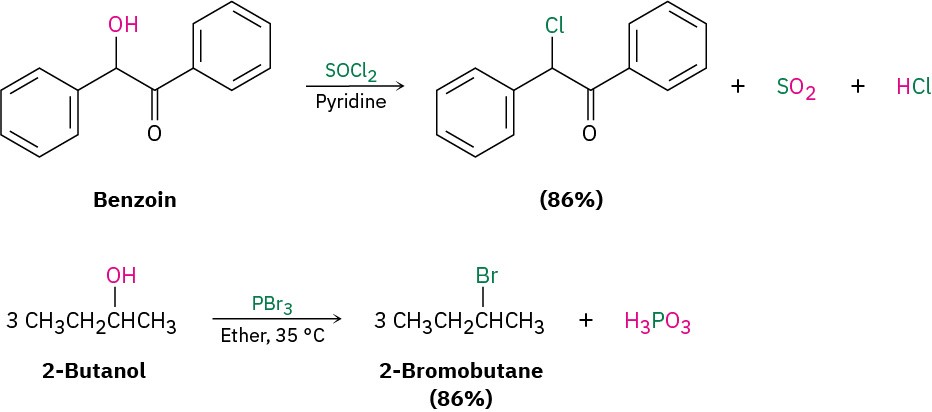
As the preceding examples indicate, the yields of these SOCl2 and PBr3 reactions are generally high and other functional groups such as ethers, carbonyls, and aromatic rings don’t usually interfere. We’ll look at the mechanisms of these and other related substitution reactions in Section 7.3.
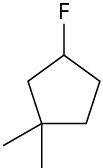
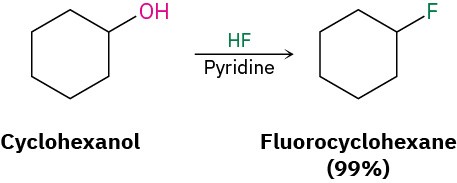
Alkyl fluorides can also be prepared from alcohols. Numerous alternative reagents are used for such reactions, including diethylaminosulfur trifluoride [(CH3CH2)2NSF3] and HF in pyridine solvent.
 Problem 6.3
Problem 6.3
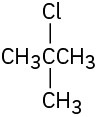
How would you prepare the following alkyl halides from the corresponding alcohols?
(a)
(b)
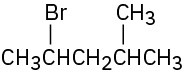
(c)
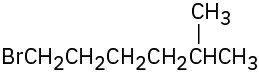
(d)