11.4 Synthesis of Carboxylic Acids
Let’s review briefly some of the methods for preparing carboxylic acids that we’ve seen in previous chapters.
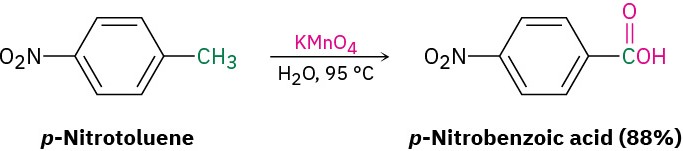
- Oxidation of a substituted alkylbenzene with KMnO4 gives a substituted benzoic acid (Section 8.8). Both primary and secondary alkyl groups can be oxidized, but tertiary groups are not affected.
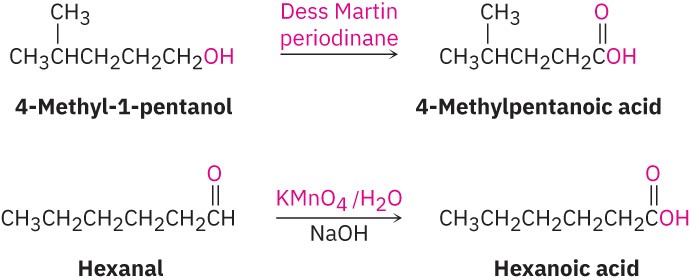
- Oxidation of a primary alcohol or an aldehyde yields a carboxylic acid (Section 9.8 and Section 10.3). Primary alcohols are often oxidized with the Dess Martin periodinane, and aldehydes are similarly oxidized with alkaline KMnO4.
Hydrolysis of Nitriles
Carboxylic acids can be prepared from nitriles on heating with aqueous acid or base by a mechanism that we’ll discuss later in Chapter 11. Since nitriles themselves are usually made by SN2 reaction of a primary or secondary alkyl halide with CN–, the two-step sequence of cyanide displacement followed by nitrile hydrolysis is a good way to make a carboxylic acid from an alkyl halide (RBr → RC≡N → RCO2H). Note that the product acid has one more carbon than the starting alkyl halide. One example occurs in a commercial route for the synthesis of the nonsteroidal anti-inflammatory drug ibuprofen. (See Chapter 8 Chemistry Matters.)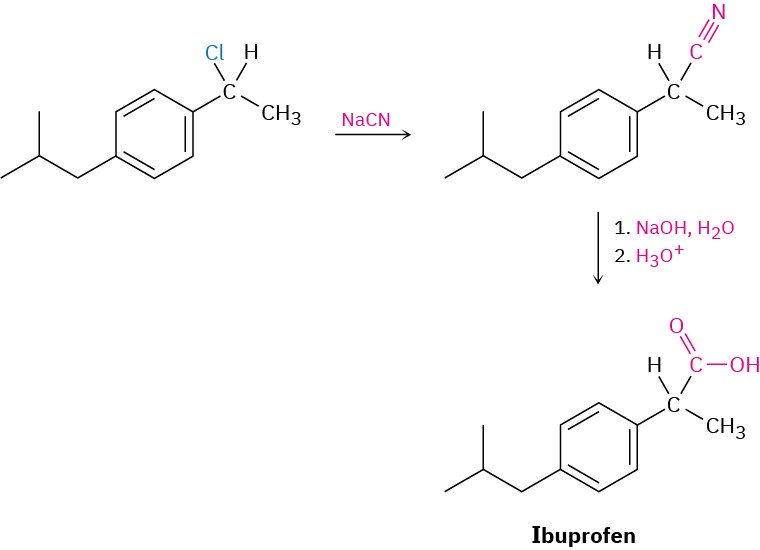 Problem 11.9
Problem 11.9
How would you prepare CH3CH2CH2CO2H from CH3CH2CH2Br?

