9.2 Properties of Alcohols and Phenols
Alcohols and phenols have nearly the same geometry around the oxygen atom as water. The R–O–H bond angle has an approximately tetrahedral value (108.5° in methanol, for instance), and the oxygen atom is sp3-hybridized.
Also like water, alcohols and phenols have higher boiling points than might be expected, because of hydrogen-bonding (Section 1.22). A positively polarized –OH hydrogen atom from one molecule is attracted to a lone pair of electrons on the electronegative oxygen atom of another molecule, resulting in a weak force that holds the molecules together (Figure 9.3). These intermolecular attractions must be overcome for a molecule to break free from the liquid and enter the vapor state, so the boiling temperature is raised. For example, 1-propanol (MW = 60), butane (MW = 58), and chloroethane (MW = 65) have similar molecular weights, yet 1-propanol boils at 97 °C, compared with –0.5 °C for the alkane and 12.5 °C for the chloroalkane.
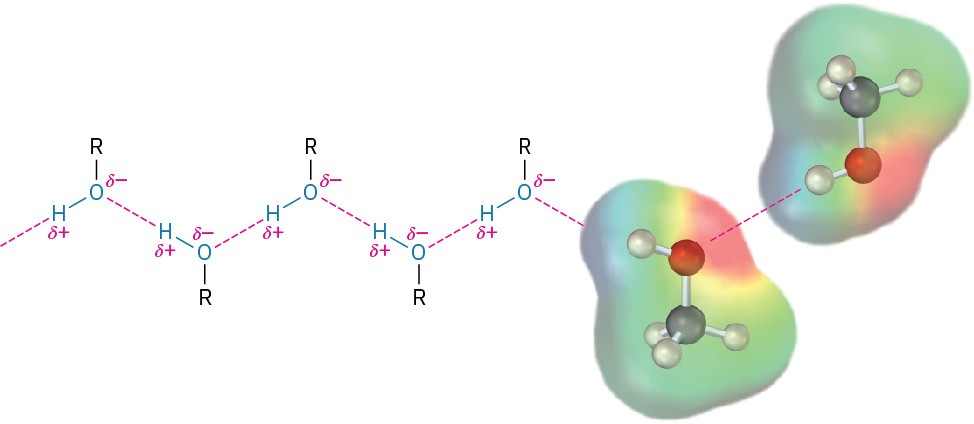 Figure 9.3 Hydrogen-bonding in alcohols and phenols. Attraction between a positively polarized –OH hydrogen and a negatively polarized oxygen holds molecules together. The electrostatic potential map of methanol shows the positively polarized –OH hydrogen and the negatively polarized oxygen.
Figure 9.3 Hydrogen-bonding in alcohols and phenols. Attraction between a positively polarized –OH hydrogen and a negatively polarized oxygen holds molecules together. The electrostatic potential map of methanol shows the positively polarized –OH hydrogen and the negatively polarized oxygen.
Another similarity with water is that alcohols and phenols are both weakly basic and weakly acidic. As weak bases, they are reversibly protonated by strong acids to yield oxonium ions, ROH2+.
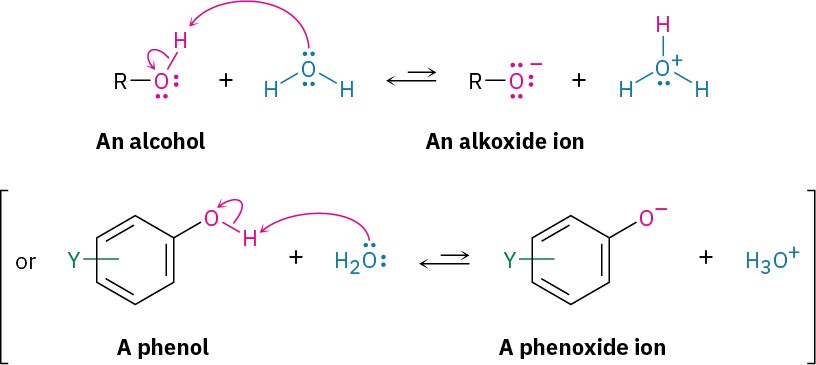
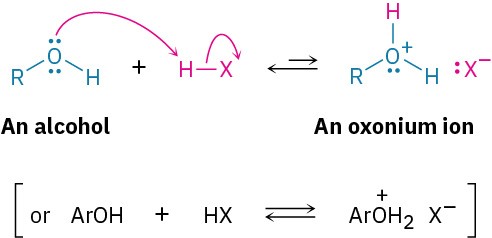 As weak acids, they dissociate slightly in dilute aqueous solution by donating a proton to water, generating H3O+ and an alkoxide ion, RO–, or a phenoxide ion, ArO–.
As weak acids, they dissociate slightly in dilute aqueous solution by donating a proton to water, generating H3O+ and an alkoxide ion, RO–, or a phenoxide ion, ArO–.
 Recall from the earlier discussion of acidity in Section 1.17 to Section 1.21 that the strength of any acid HA in water can be expressed by an acidity constant, Ka.
Recall from the earlier discussion of acidity in Section 1.17 to Section 1.21 that the strength of any acid HA in water can be expressed by an acidity constant, Ka.
𝐾a =([A–][H3O+]) / [HA]
p𝐾a = −log 𝐾a
Compounds with a smaller Ka and larger pKa are less acidic, whereas compounds with a larger Ka and smaller pKa are more acidic. As shown in Table 9.1, simple alcohols like methanol and ethanol are about as acidic as water, but the more highly substituted tert-butyl alcohol is somewhat weaker. Substituent groups also have a significant effect: 2,2,2-trifluoroethanol is approximately 3700 times stronger than ethanol, for instance. Phenols and thiols, the sulfur analogs of alcohols, are substantially more acidic than water.
Table 9.1 Acidity Constants of Some Alcohols and Phenols
|
Compound |
pKa |
|
|
(CH3)3COH |
18 |
|
|
CH3CH2OH |
16 | |
|
H2O |
15.74 | |
|
CH3OH |
15.54 | |
|
CF3CH2OH |
12.43 | |
|
p-Aminophenol |
10.46 | |
|
CH3SH |
10.3 | |
|
p-Methylphenol |
10.17 | |
|
Phenol |
9.89 | |
|
p-Chlorophenol |
9.38 | |
|
p-Nitrophenol |
7.15 | |
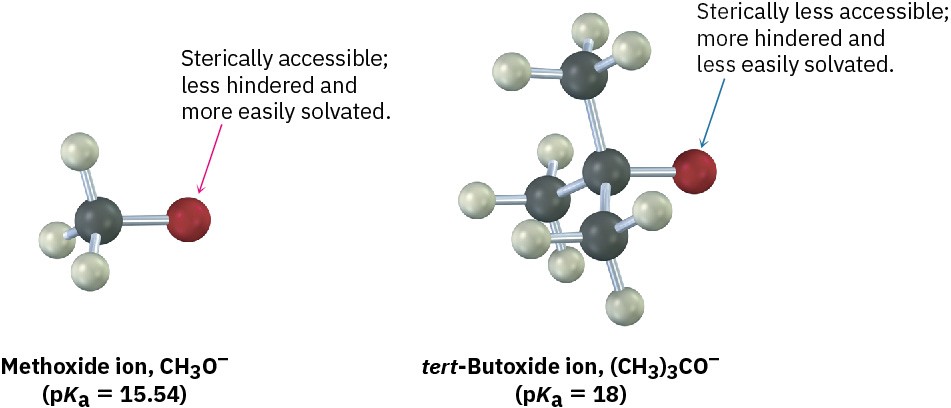
The effect of alkyl substitution on alcohol acidity is due primarily to solvation of the alkoxide ion formed on acid dissociation. The more readily the alkoxide ion is solvated by water, the more stable it is, the more its formation is energetically favored, and the greater the acidity of the parent alcohol. For example, the oxygen atom of an unhindered alkoxide ion, such as that from methanol, is sterically accessible and is easily solvated by water. The oxygen atom of a hindered alkoxide ion, however, such as that from tert-butyl alcohol, is less easily solvated and is therefore less stable.
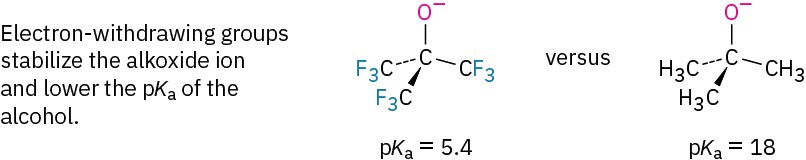
 Inductive effects (Section 8.6) are also important in determining alcohol acidities. Electron-withdrawing halogen substituents, for instance, stabilize an alkoxide ion by spreading the charge over a larger volume, thus making the alcohol more acidic. Compare, for instance, the acidities of ethanol (pKa = 16) and 2,2,2-trifluoroethanol (pKa = 12.43), or of tert-butyl alcohol (pKa = 18) and nonafluoro-tert-butyl alcohol (pKa = 5.4).
Inductive effects (Section 8.6) are also important in determining alcohol acidities. Electron-withdrawing halogen substituents, for instance, stabilize an alkoxide ion by spreading the charge over a larger volume, thus making the alcohol more acidic. Compare, for instance, the acidities of ethanol (pKa = 16) and 2,2,2-trifluoroethanol (pKa = 12.43), or of tert-butyl alcohol (pKa = 18) and nonafluoro-tert-butyl alcohol (pKa = 5.4).
 Because alcohols are weak acids, they don’t react with weak bases, such as amines or bicarbonate ion, and they only react to a limited extent with metal hydroxides such as NaOH. Alcohols do, however, react with alkali metals and with strong bases such as sodium hydride (NaH), sodium amide (NaNH2), and Grignard reagents (RMgX). Alkoxides are themselves bases that are frequently used as reagents in organic chemistry. They are named systematically by adding the –ate suffix to the name of the alcohol. Methanol becomes methanolate, for instance.
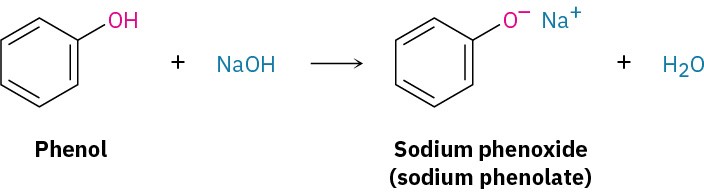
Because alcohols are weak acids, they don’t react with weak bases, such as amines or bicarbonate ion, and they only react to a limited extent with metal hydroxides such as NaOH. Alcohols do, however, react with alkali metals and with strong bases such as sodium hydride (NaH), sodium amide (NaNH2), and Grignard reagents (RMgX). Alkoxides are themselves bases that are frequently used as reagents in organic chemistry. They are named systematically by adding the –ate suffix to the name of the alcohol. Methanol becomes methanolate, for instance.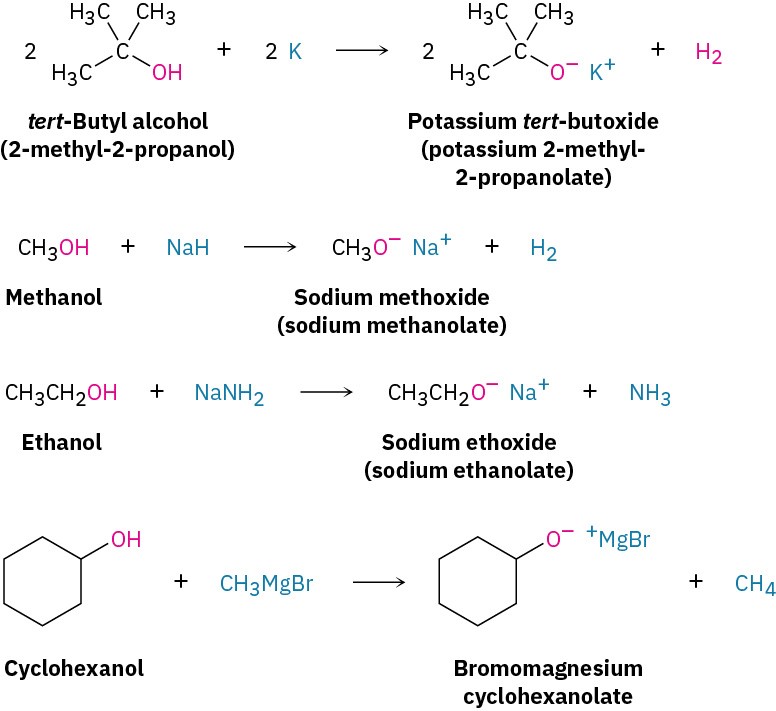 Phenols are about a million times more acidic than alcohols (Table 9.1). They are therefore soluble in dilute aqueous NaOH and can often be separated from a mixture simply by basic extraction into aqueous solution, followed by reacidification.
Phenols are about a million times more acidic than alcohols (Table 9.1). They are therefore soluble in dilute aqueous NaOH and can often be separated from a mixture simply by basic extraction into aqueous solution, followed by reacidification.
Phenols are more acidic than alcohols because the phenoxide anion is resonance-stabilized. Delocalization of the negative charge over the ortho and para positions of the aromatic ring results in increased stability of the phenoxide anion relative to undissociated phenol and in a consequently lower ∆G° for dissociation. Figure 9.4 compares electrostatic potential maps of an alkoxide ion (CH3O–) with phenoxide ion to show how the negative charge in phenoxide ion is delocalized from oxygen to the ring.
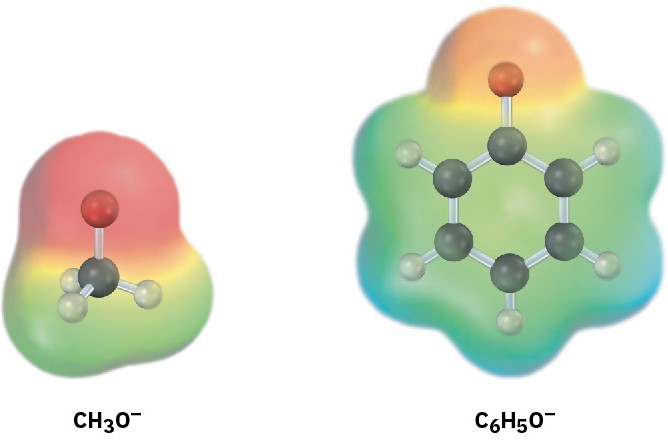
Figure 9.4 The resonance-stabilized phenoxide ion is more stable than an alkoxide ion. Electrostatic potential maps show how the negative charge is concentrated on oxygen in the methoxide ion but is spread over the aromatic ring in the phenoxide ion.
Substituted phenols can be either more acidic or less acidic than phenol itself, depending on whether the substituent is electron-withdrawing or electron-donating (Section 8.6). Phenols with an electron-withdrawing substituent are more acidic because these substituents delocalize the negative charge; phenols with an electron-donating substituent are less acidic because these substituents concentrate the charge. The acidifying effect of an electron-withdrawing substituent is particularly noticeable in phenols with a nitro group at the ortho or para position.
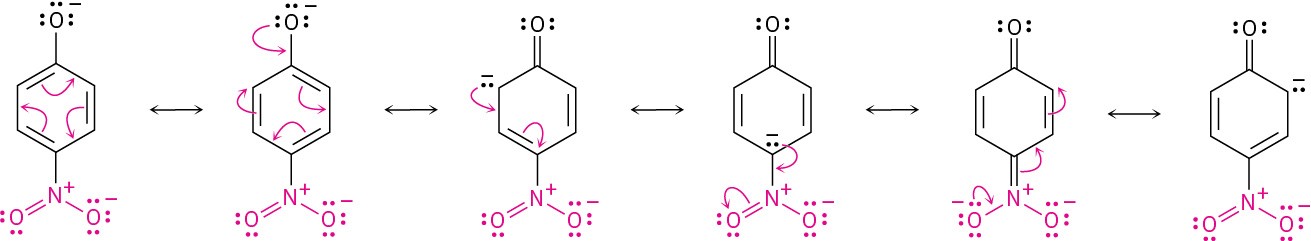
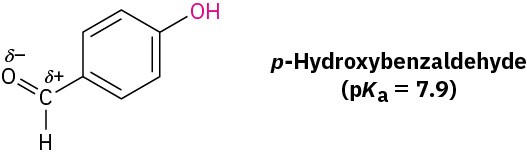
Worked Example 9.1: Predicting the Relative Acidity of a Substituted Phenol
Is p-hydroxybenzaldehyde more acidic or less acidic than phenol?
Strategy
Identify the substituent on the aromatic ring, and decide whether it is electron-donating or electron-withdrawing. Electron-withdrawing substituents make the phenol more acidic by stabilizing the phenoxide anion, and electron-donating substituents make the phenol less acidic by destabilizing the anion.
Solution
We saw in Section 8.6 that a carbonyl group is electron-withdrawing. Thus, p-hydroxybenzaldehyde is more acidic (pKa = 7.9) than phenol (pKa = 9.89).
 Problem 9.3
Problem 9.3
The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point with increasing substitution of the OH-bearing carbon. How might you account for this trend?
- 1-Butanol, bp 117.5 °C
- 2-Butanol, bp 99.5 °C
- 2-Methyl-2-propanol, bp 82.2 °C
Problem 9.4
Rank the following substances in order of increasing acidity:
(a) (CH3)2CHOH, HC≡CH, (CF3)2CHOH, CH3OH
(b) Phenol, p-methylphenol, p-(trifluoromethyl)phenol


