5.7 Radical Additions to Alkenes: Chain-Growth Polymers
Recall that radicals can add to C═C bonds, taking one electron from the double bond and leaving one behind to yield a new radical. Let’s now look at the process in more detail, focusing on the industrial synthesis of alkene polymers. A polymer is a large—sometimes very large—molecule, built up by repetitive joining together of many smaller molecules, called monomers.
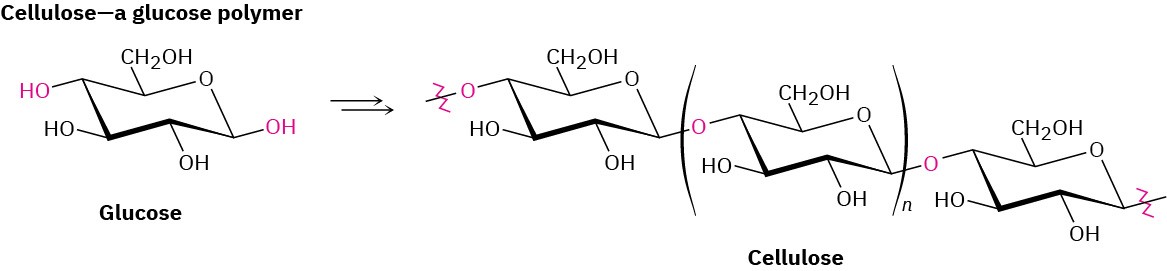
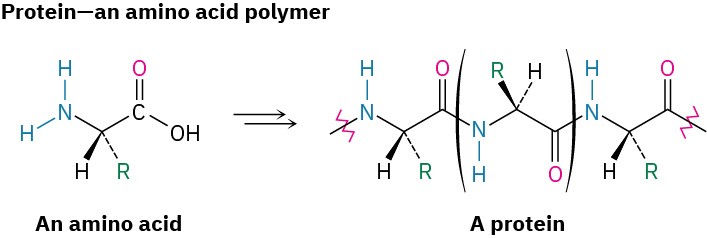
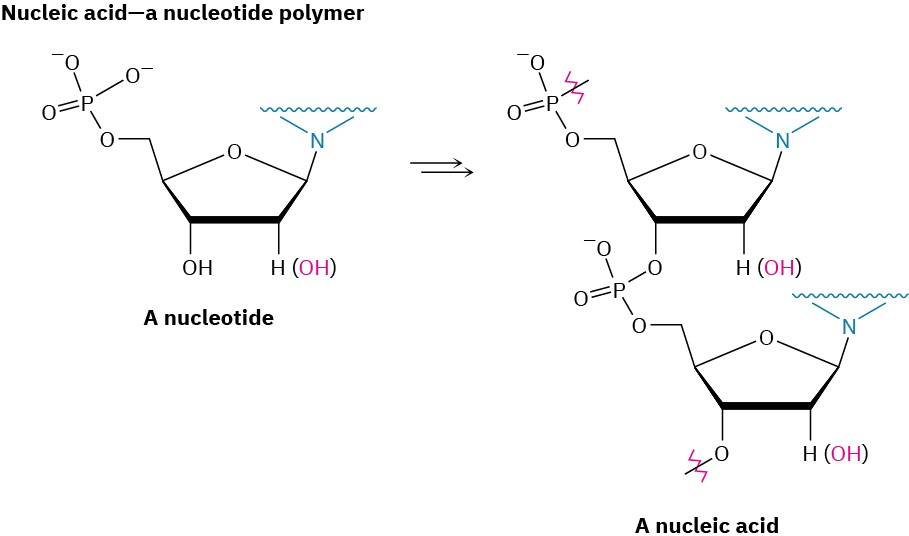
Nature makes wide use of biological polymers. Cellulose, for instance, is a polymer built of repeating glucose monomer units; proteins are polymers built of repeating amino acid monomers; and nucleic acids are polymers built of repeating nucleotide monomers.
Synthetic polymers, such as polyethylene, are much simpler chemically than biopolymers, but there is still a great diversity to their structures and properties, depending on the identity of the monomers and on the reaction conditions used for polymerization. The simplest synthetic polymers are those that result when an alkene is treated with a small amount of a suitable catalyst. Ethylene, for example, yields polyethylene, an enormous alkane that may have a molecular weight up to 6 million u and may contain as many as 200,000 monomer units. Worldwide production of polyethylene is approximately 88 million tons per year.
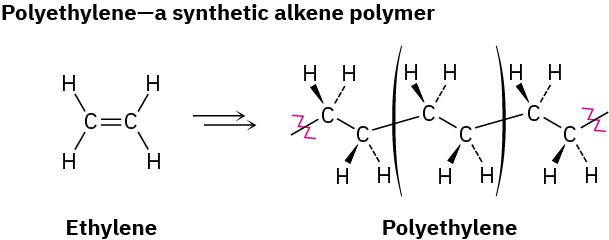 Polyethylene and other simple alkene polymers are called chain-growth polymers because they are formed in a chain-reaction process in which an initiator adds to a carbon–carbon double bond to yield a reactive intermediate. The intermediate then reacts with a second molecule of monomer to yield a new intermediate, which reacts with a third monomer unit, and so on.
Polyethylene and other simple alkene polymers are called chain-growth polymers because they are formed in a chain-reaction process in which an initiator adds to a carbon–carbon double bond to yield a reactive intermediate. The intermediate then reacts with a second molecule of monomer to yield a new intermediate, which reacts with a third monomer unit, and so on.
Historically, ethylene polymerization was carried out at high pressure (1000–3000 atm) and high temperature (100–250°C) in the presence of a radical initiator such as benzoyl peroxide. Like many radical reactions, the mechanism of ethylene polymerization occurs in three steps: initiation, propagation, and termination:
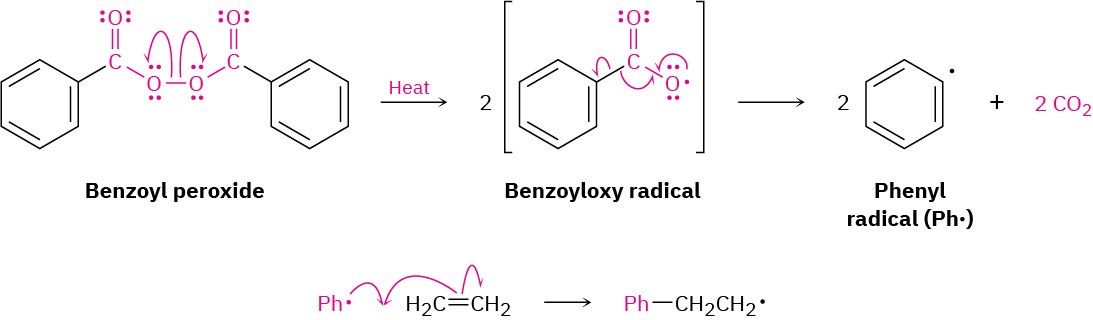
Initiation: The polymerization reaction is initiated when a few radicals are generated on heating a small amount of benzoyl peroxide catalyst to break the weak O−O bond. The initially formed benzoyloxy radical loses CO2 and gives a phenyl radical (Ph·), which adds to the C═C bond of ethylene to start the polymerization process. One electron from the ethylene double bond pairs up with the odd electron on the phenyl radical to form a new C−C bond, and the other electron remains on carbon.
Propagation: Polymerization occurs when the carbon radical formed in the initiation step adds to another ethylene molecule to yield another radical. Repetition of the process for hundreds or thousands of times builds the polymer chain.
![]()
Termination: The chain process is eventually ended by a reaction that consumes the radical. The combination of two growing chains is one possible chain- terminating reaction.
2 R– CH2CH2· → R– CH2CH2CH2CH2– R
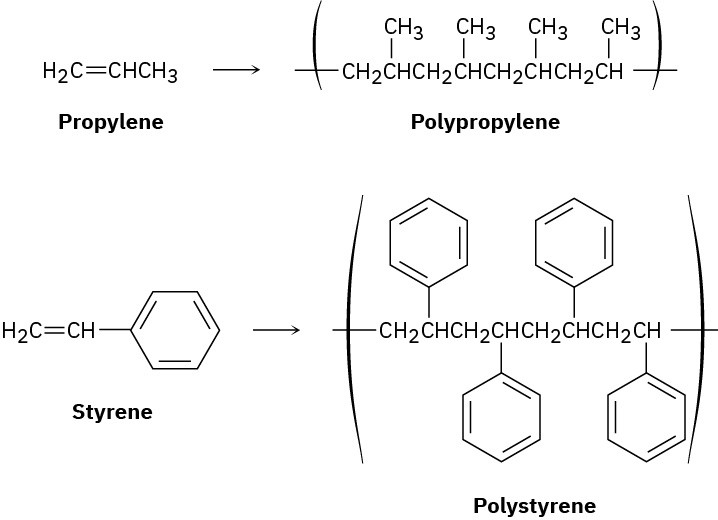
Ethylene is not unique in its ability to form a polymer. Many substituted ethylenes, called vinyl monomers, also undergo polymerization to yield polymers with substituent groups regularly spaced on alternating carbon atoms along the chain. Propylene, for example, yields polypropylene, and styrene yields polystyrene.
 When an unsymmetrically substituted vinyl monomer such as propylene or styrene is polymerized, the radical addition steps can take place at either end of the double bond to yield either a primary radical intermediate (RCH2·) or a secondary radical (R2CH·). Just as in electrophilic addition reactions, however, we find that only the more highly substituted, secondary radical is formed.
When an unsymmetrically substituted vinyl monomer such as propylene or styrene is polymerized, the radical addition steps can take place at either end of the double bond to yield either a primary radical intermediate (RCH2·) or a secondary radical (R2CH·). Just as in electrophilic addition reactions, however, we find that only the more highly substituted, secondary radical is formed.
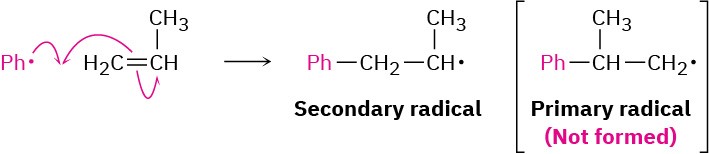
Table 5.1 shows some commercially important alkene polymers, their uses, and the monomers from which they are made.
Table 5.1 Some Alkene Polymers and Their Uses
|
Monomer |
Formula |
Trade or common name of polymer |
Uses |
|
Ethylene |
H2C═CH2 |
Polyethylene |
Packaging, bottles |
|
Propene (propylene) |
H2C═CHCH3 |
Polypropylene |
Moldings, rope, carpets |
|
Chloroethylene (vinyl chloride) |
H2C═CHCl |
Poly(vinyl chloride) |
Insulation, films, pipes |
|
Styrene |
H2C═CHC6H5 |
Polystyrene |
Foam, moldings |
|
Tetrafluoroethylene |
F3C═CF3 |
Teflon |
Gaskets, nonstick coatings |
|
Acrylonitrile |
H2C═CHCN |
Orlon, Acrilan |
Fibers |
|
Methyl methacrylate |
H2C=C(CH3)CO2CH3 |
Plexiglas, Lucite |
Paint, sheets, moldings |
|
Vinyl acetate |
H2C═CHOCOCH3 |
Poly(vinyl acetate) |
Paint, adhesives, foams |
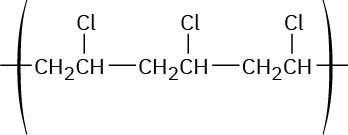
Worked Example 5.3: Predicting the Structure of a Polymer
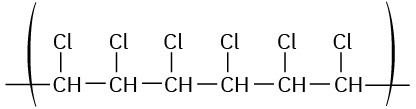
Show the structure of poly(vinyl chloride), a polymer made from H2C═CHCl, by drawing several repeating units.
Strategy
Mentally break the carbon–carbon double bond in the monomer unit, and form single bonds by connecting numerous units together.
Solution
The general structure of poly(vinyl chloride) is
Problem 5.8
Show the monomer units you would use to prepare the following polymers:
(a)
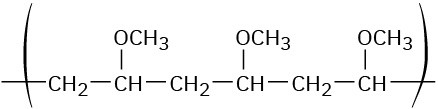
(b)