9.7 Reactions of Alcohols
We’ve already seen several reactions of alcohols—their conversion into alkyl halides and tosylates in Section 7.5 and their dehydration to give alkenes—albeit without mechanistic details. Let’s now look at those details.
Conversion of Alcohols into Alkyl Halides
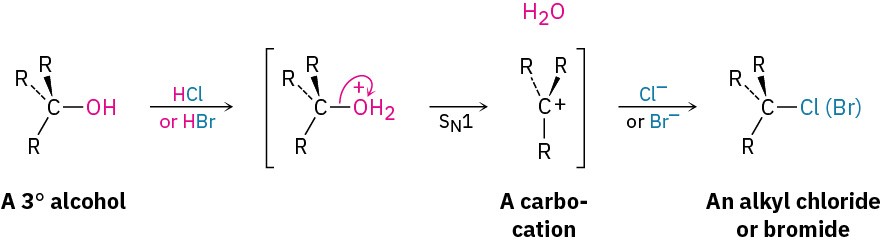
Tertiary alcohols react with either HCl or HBr at 0 °C by an SN1 mechanism through a carbocation intermediate. Primary and secondary alcohols are much more resistant to acid, however, and are best converted into halides by treatment with either SOCl2 or PBr3 through an SN2 mechanism.
The reaction of a tertiary alcohol with HX takes place by an SN1 mechanism when acid protonates the hydroxyl oxygen atom. Water is expelled to generate a carbocation, and the cation reacts with nucleophilic halide ion to give the alkyl halide product.
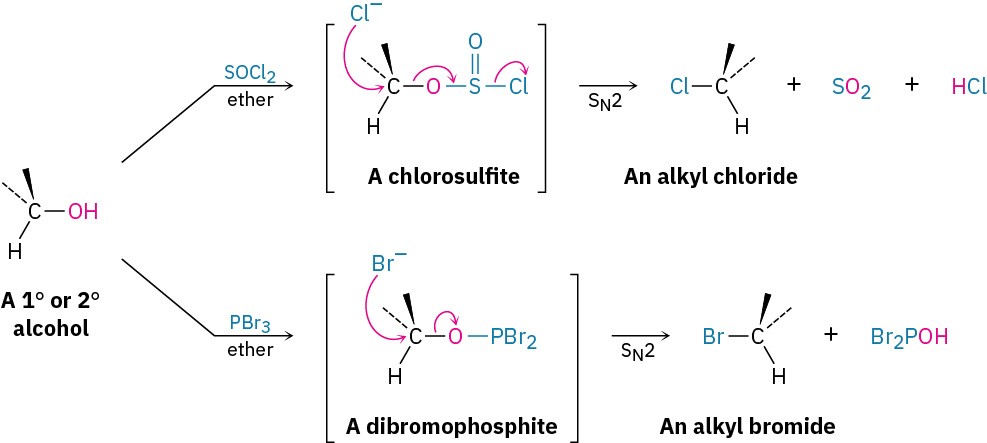
The reactions of primary and secondary alcohols with SOCl2 and PBr3 take place by SN2 mechanisms. Hydroxide ion itself is too poor a leaving group to be displaced by nucleophiles in SN2 reactions, but reaction of an alcohol with SOCl2 or PBr3 converts the – OH into a much better leaving group, either a chlorosulfite (–OSOCl) or a dibromophosphite (–OPBr2), which is readily expelled by backside nucleophilic substitution.
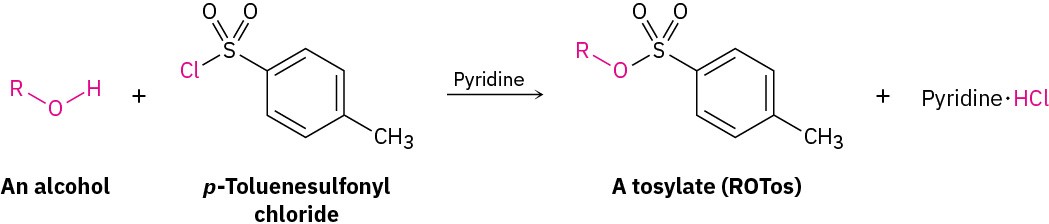
Conversion of Alcohols into Tosylates
Alcohols react with p-toluenesulfonyl chloride (tosyl chloride, p-TosCl) in pyridine solution to yield alkyl tosylates, ROTos (Section 7.3). Only the O–H bond of the alcohol is broken in this reaction; the C–O bond remains intact, so no change of configuration occurs if the oxygen is attached to a chirality center. The resultant alkyl tosylates behave much like alkyl halides, undergoing both SN1 and SN2 substitution reactions.
 One of the most important reasons for using tosylates in SN2 reactions is stereochemical. The SN2 reaction of an alcohol with an alkyl halide proceeds with two inversions of configuration—one to make the halide from the alcohol and one to substitute the halide— and yields a product with the same stereochemistry as the starting alcohol. The SN2 reaction of an alcohol with a tosylate, however, proceeds with only one inversion and yields a product of opposite stereochemistry to the starting alcohol. Figure 9.6 shows a series of reactions on the R enantiomer of 2-octanol that illustrates these stereochemical relationships.
One of the most important reasons for using tosylates in SN2 reactions is stereochemical. The SN2 reaction of an alcohol with an alkyl halide proceeds with two inversions of configuration—one to make the halide from the alcohol and one to substitute the halide— and yields a product with the same stereochemistry as the starting alcohol. The SN2 reaction of an alcohol with a tosylate, however, proceeds with only one inversion and yields a product of opposite stereochemistry to the starting alcohol. Figure 9.6 shows a series of reactions on the R enantiomer of 2-octanol that illustrates these stereochemical relationships.
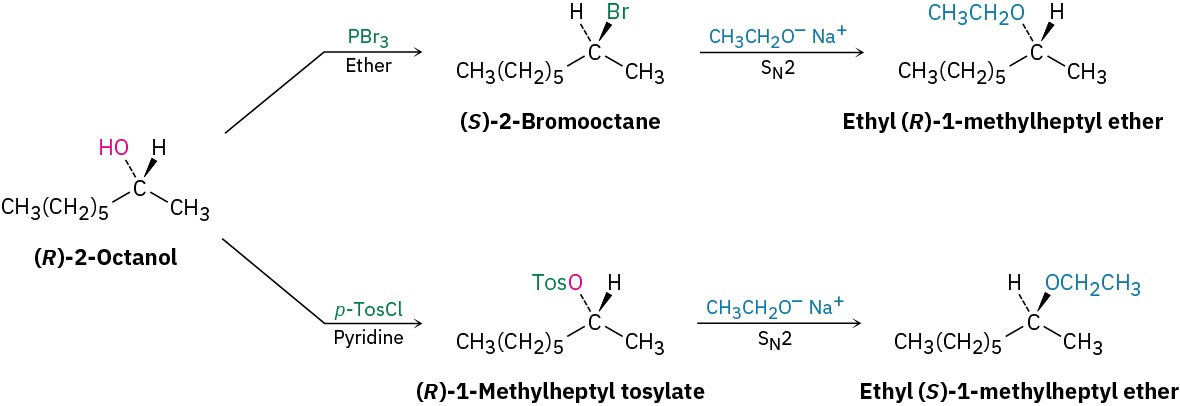 Figure 9.6 Stereochemical consequences of SN2 reactions on derivatives of (R)-2-octanol.
Figure 9.6 Stereochemical consequences of SN2 reactions on derivatives of (R)-2-octanol.
Substitution through the halide gives a product with the same stereochemistry as the starting alcohol; substitution through the tosylate gives a product with opposite stereochemistry to the starting alcohol.
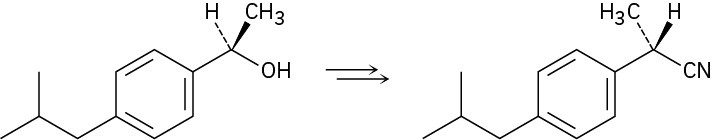
Problem 9.11
How would you carry out the following transformation, a step used in the commercial synthesis of (S)-ibuprofen?
Dehydration of Alcohols to Yield Alkenes
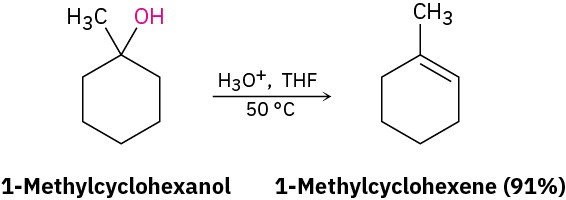
A third important reaction of alcohols, both in the laboratory and in biological pathways, is their dehydration to give alkenes. Because of the usefulness of the reaction, a number of methods have been devised for carrying out dehydrations. One method that works particularly well for tertiary alcohols is the acid-catalyzed reaction discussed throughout Chapter 5. For example, treatment of 1-methylcyclohexanol with warm, aqueous sulfuric acid in a solvent such as tetrahydrofuran results in loss of water and formation of 1-methylcyclohexene.
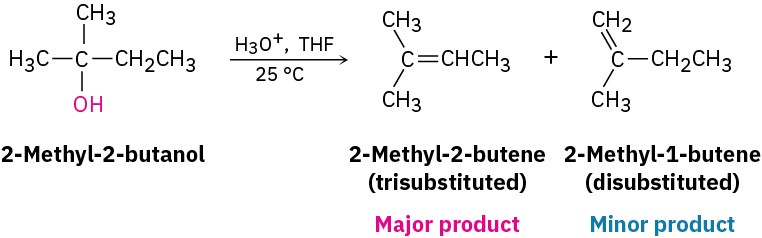
 Acid-catalyzed dehydrations usually follow Zaitsev’s rule (Section 7.7) and yield the more stable alkene as the major product. Thus, 2-methyl-2-butanol gives primarily 2-methyl-2-butene (trisubstituted double bond) rather than 2-methyl-1-butene (disubstituted double bond).
Acid-catalyzed dehydrations usually follow Zaitsev’s rule (Section 7.7) and yield the more stable alkene as the major product. Thus, 2-methyl-2-butanol gives primarily 2-methyl-2-butene (trisubstituted double bond) rather than 2-methyl-1-butene (disubstituted double bond).
 This reaction is an E1 process (Section 7.10) and occurs by the three-step mechanism shown in Figure 9.7 below. Protonation of the alcohol oxygen is followed by unimolecular loss of water to generate a carbocation intermediate and final loss of a proton from the neighboring carbon atom to complete the process. As with most E1 reactions, tertiary alcohols react fastest because they lead to stabilized, tertiary carbocation intermediates. Secondary alcohols can be made to react, but the conditions are severe (75% H2SO4, 100 °C) and sensitive molecules don’t survive.
This reaction is an E1 process (Section 7.10) and occurs by the three-step mechanism shown in Figure 9.7 below. Protonation of the alcohol oxygen is followed by unimolecular loss of water to generate a carbocation intermediate and final loss of a proton from the neighboring carbon atom to complete the process. As with most E1 reactions, tertiary alcohols react fastest because they lead to stabilized, tertiary carbocation intermediates. Secondary alcohols can be made to react, but the conditions are severe (75% H2SO4, 100 °C) and sensitive molecules don’t survive.
Figure 9.7 MECHANISM: Mechanism for the acid-catalyzed dehydration of a tertiary alcohol to yield an alkene. The process is an E1 reaction and involves a carbocation intermediate.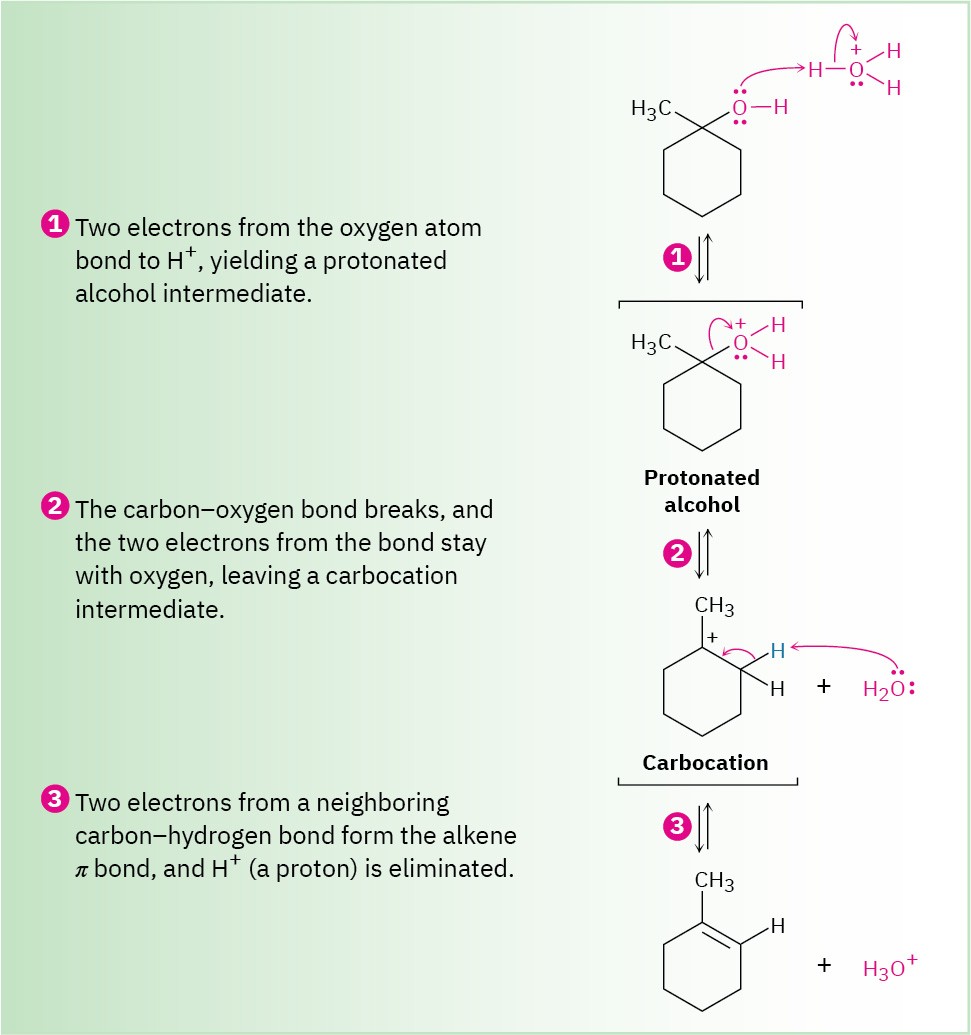
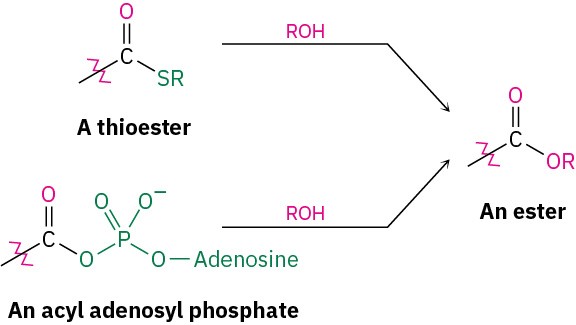
Conversion of Alcohols into Esters
Alcohols react with carboxylic acids to give esters, a reaction that is common in both the laboratory and living organisms. In the laboratory, the reaction can be carried out in a single step if a strong acid is used as catalyst. More frequently, though, the reactivity of the carboxylic acid is enhanced by first converting it into a carboxylic acid chloride, which then reacts with the alcohol.
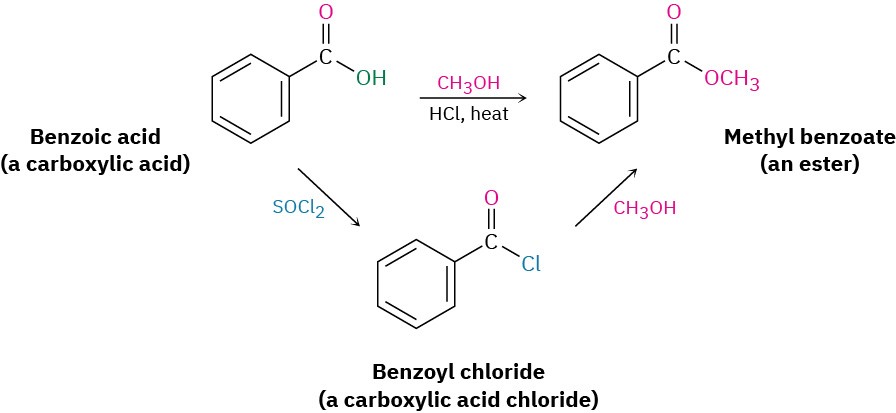 In living organisms, a similar process occurs, though a thioester or acyl adenosyl phosphate acts as substrate rather than a carboxylic acid chloride.
In living organisms, a similar process occurs, though a thioester or acyl adenosyl phosphate acts as substrate rather than a carboxylic acid chloride.