5.10 Reactions of Alkynes: Addition of HX and X2
You might recall from Section 1.9 that a carbon–carbon triple bond results from the interaction of two sp-hybridized carbon atoms. The two sp hybrid orbitals of carbon lie at an angle of 180° to each other along an axis perpendicular to the axes of the two unhybridized 2py and 2pz orbitals. When two sp-hybridized carbons approach each other, one sp–sp σ bond and two p–p π bonds are formed. The two remaining sp orbitals form bonds to other atoms at an angle of 180° from the carbon–carbon bond. Thus, acetylene is a linear molecule with H–C≡C bond angles of 180° (Figure 5.13). The length of the C≡C bond is 120 pm, and its strength is approximately 965 kJ/mol (231 kcal/mol), making it the shortest and strongest known carbon–carbon bond.
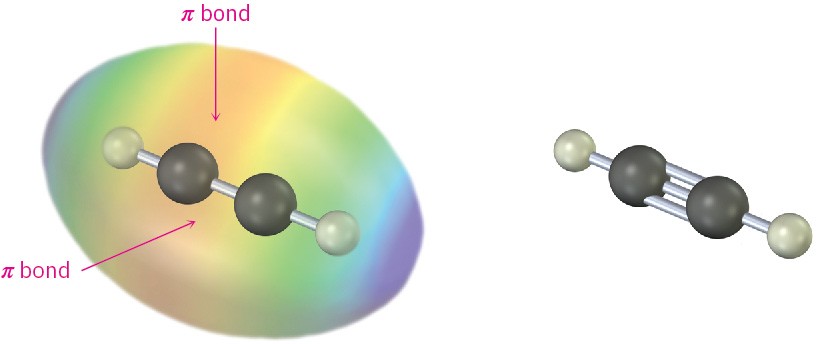 Figure 5.13 The structure of acetylene, H–C≡C–H. The H–C≡C bond angles are 180°, and the C≡C bond length is 120 pm. The electrostatic potential map shows that the π bonds create a negative belt around the molecule.
Figure 5.13 The structure of acetylene, H–C≡C–H. The H–C≡C bond angles are 180°, and the C≡C bond length is 120 pm. The electrostatic potential map shows that the π bonds create a negative belt around the molecule.
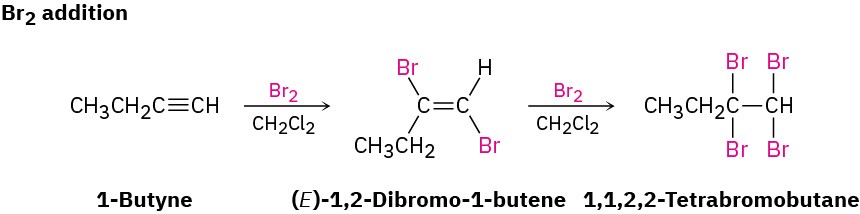
As a general rule, electrophiles undergo addition reactions with alkynes much as they do with alkenes. Take the reaction of alkynes with HX, for instance. The reaction often can be stopped with the addition of 1 equivalent of HX, but reaction with an excess of HX leads to a dihalide product. For example, reaction of 1-hexyne with 2 equivalents of HBr yields 2,2-dibromohexane. As the following examples indicate, the regiochemistry of addition follows Markovnikov’s rule, with halogen adding to the more highly substituted side of the alkyne bond and hydrogen adding to the less highly substituted side. Trans stereochemistry of H and X normally, although not always, occurs in the product.
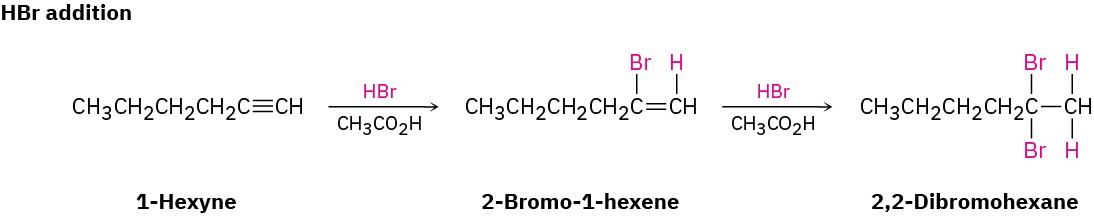
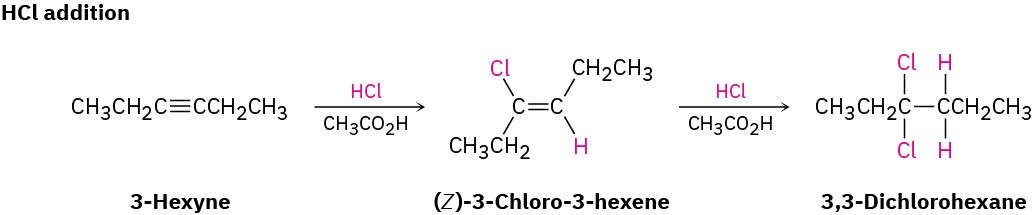
Bromine and chlorine also add to alkynes to give addition products, and trans stereochemistry again results.
 Problem 5.12
Problem 5.12
What products would you expect from the following reactions?
(a) 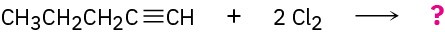
(b)
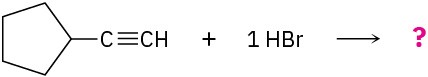
(c)