12.5 Reactions of Amines
Alkylation and Acylation
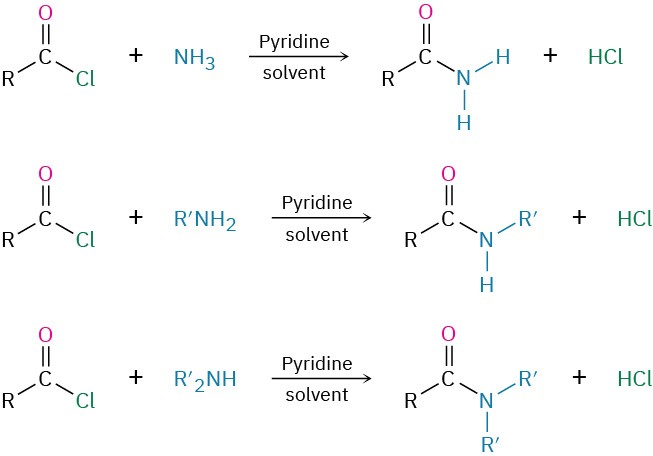
We’ve already studied the two most general reactions of amines—alkylation and acylation. As we saw earlier in this chapter, primary, secondary, and tertiary amines can be alkylated by reaction with a primary alkyl halide. Alkylations of primary and secondary amines are difficult to control and often give mixtures of products, but tertiary amines are cleanly alkylated to give quaternary ammonium salts. Primary and secondary (but not tertiary) amines can also be acylated by nucleophilic acyl substitution reaction with an acid chloride or an acid anhydride to yield an amide (Section 11.7 and Section 11.8). Note that overacylation of the nitrogen does not occur because the amide product is much less nucleophilic and less reactive than the starting amine.
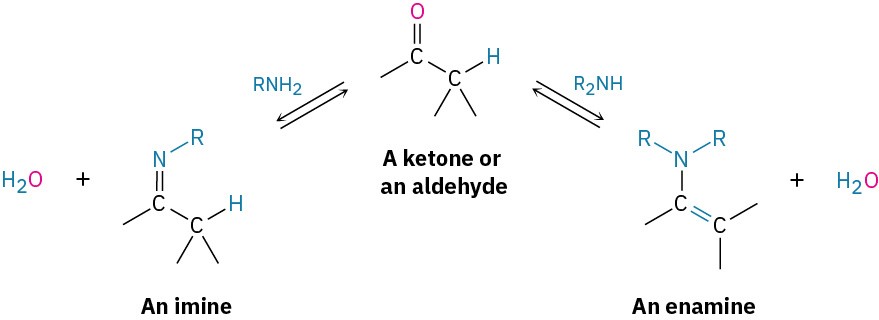
In addition, primary amines react with aldehydes and ketones to form imines and secondary amines react with aldehydes and ketones to form enamines .