9.14 Reactions of Epoxides: Ring-Opening
Acid-Catalyzed Epoxide Opening
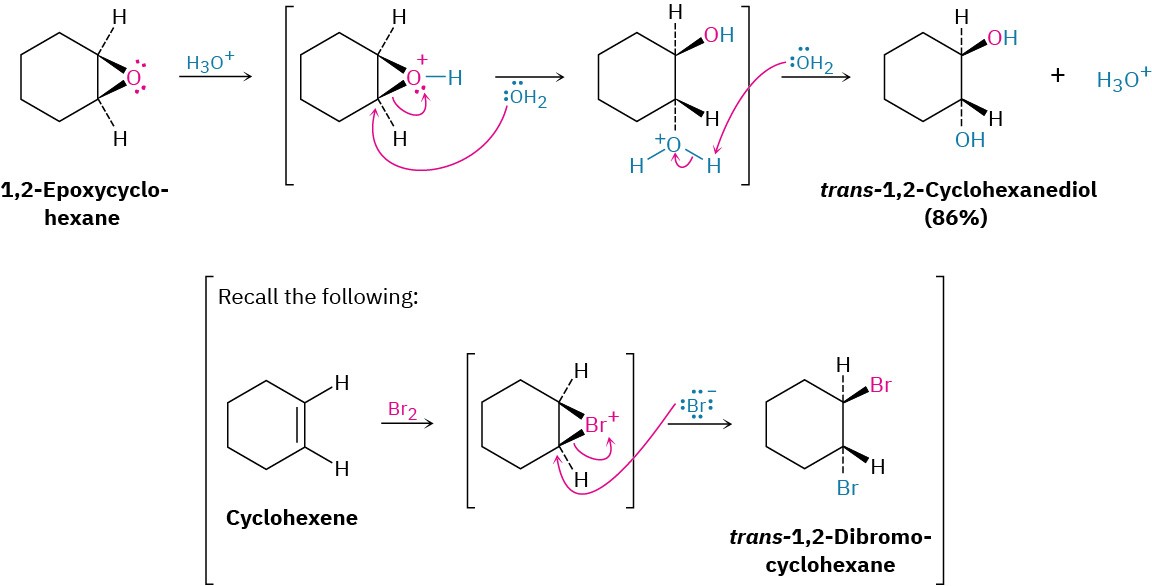
Epoxides are cleaved by treatment with acid just as other ethers are, but under much milder conditions because of ring strain. As we saw in Section 5.6, dilute aqueous acid at room temperature is sufficient for facilitating the hydrolysis of epoxides to give 1,2-diols, also called vicinal glycols. (The word vicinal means “adjacent,” and a glycol is a diol.) The epoxide cleavage takes place by SN2-like backside attack of a nucleophile on the protonated epoxide, giving a trans-1,2-diol as product.
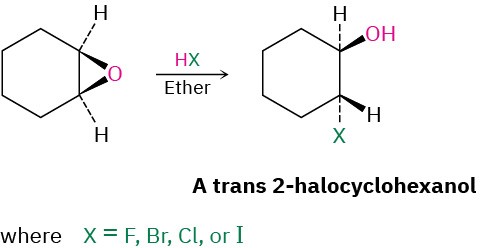
 Epoxides can also be opened by reaction with acids other than H3O+. If anhydrous HX is used, for instance, an epoxide is converted into a trans halohydrin.
Epoxides can also be opened by reaction with acids other than H3O+. If anhydrous HX is used, for instance, an epoxide is converted into a trans halohydrin.
Base-Catalyzed Epoxide Opening
Unlike other ethers, epoxide rings can be cleaved by bases and nucleophiles as well as by acid. Although an ether oxygen is normally a poor leaving group in an SN2 reaction (Section 7.3), the strain of the three-membered ring causes epoxides to react with hydroxide ion at elevated temperatures.
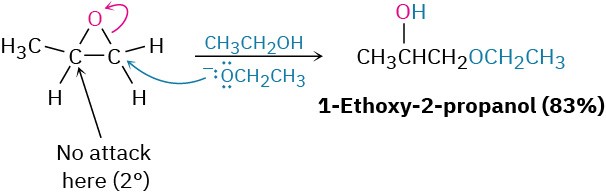
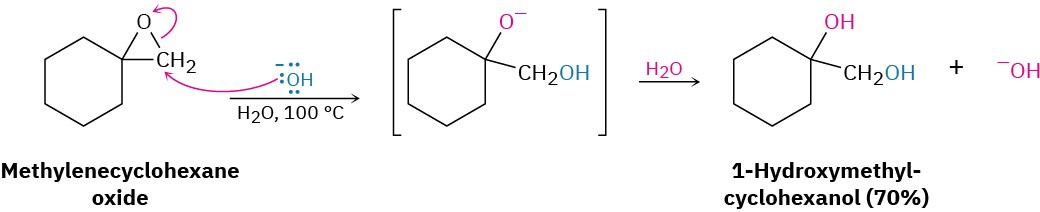 Base-catalyzed epoxide opening is a typical SN2 reaction in which attack of the nucleophile takes place at the less hindered epoxide carbon. For example, 1,2-epoxypropane reacts with ethoxide ion exclusively at the less highly substituted, primary carbon to give 1- ethoxy-2-propanol.
Base-catalyzed epoxide opening is a typical SN2 reaction in which attack of the nucleophile takes place at the less hindered epoxide carbon. For example, 1,2-epoxypropane reacts with ethoxide ion exclusively at the less highly substituted, primary carbon to give 1- ethoxy-2-propanol.
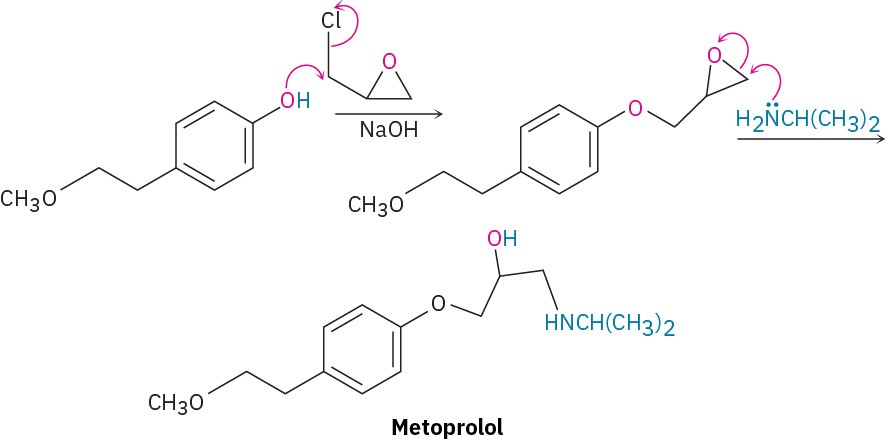
 Many different nucleophiles can be used for epoxide opening, including amines (RNH2 or R2NH) and Grignard reagents (RMgX). An example of an amine reacting with an epoxide occurs in the commercial synthesis of metoprolol, a so-called β-blocker that is used for treatment of cardiac arrhythmias, hypertension, and heart attacks. β-Blockers are among the most widely prescribed drugs in the world.
Many different nucleophiles can be used for epoxide opening, including amines (RNH2 or R2NH) and Grignard reagents (RMgX). An example of an amine reacting with an epoxide occurs in the commercial synthesis of metoprolol, a so-called β-blocker that is used for treatment of cardiac arrhythmias, hypertension, and heart attacks. β-Blockers are among the most widely prescribed drugs in the world.