11.2 Occurrence and Properties of Carboxylic Acids and Derivatives
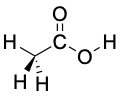
Carboxylic acids are similar in some respects to both ketones and alcohols. Like ketones, the carboxyl carbon is sp2-hybridized, and carboxylic acid groups are therefore planar with C–C═O and O═C–O bond angles of approximately 120° (Table 11.3).
Table 11.3 Physical Parameters for Acetic Acid
 |
|||
|
(degrees) |
Bond length |
(pm) |
|
|
C–C═O |
119 |
C–C |
152 |
|
C–C–OH |
119 |
C═O |
125 |
|
O═C–OH |
122 |
C–OH |
131 |
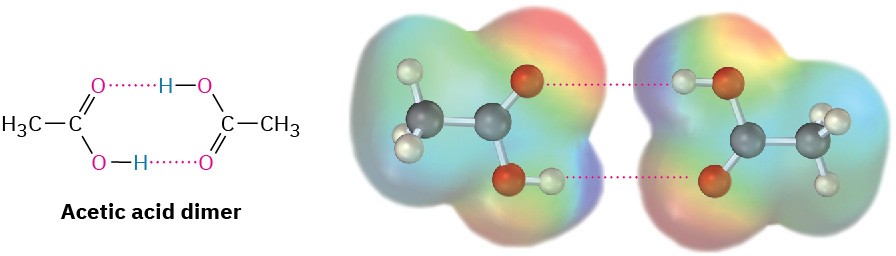
Like alcohols, carboxylic acids are strongly associated because of hydrogen-bonding. Most carboxylic acids exist as cyclic dimers held together by two hydrogen bonds. This strong hydrogen-bonding has a noticeable effect on boiling points, making carboxylic acids boil far less easily than their corresponding alcohols. Acetic acid, for instance, has a boiling point of 117.9 °C, versus 78.3 °C for ethanol, even though both compounds have two carbons.
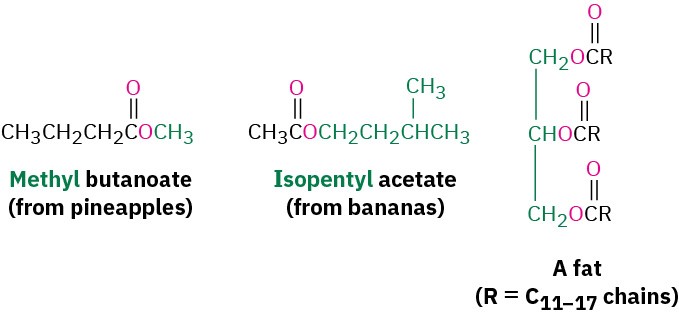
 Esters are among the most widespread of all naturally occurring compounds. Many simple esters are pleasant-smelling liquids that are responsible for the fragrant odors of fruits and flowers. For example, methyl butanoate is found in pineapple oil, and isopentyl acetate is a constituent of banana oil. The ester linkage is also present in animal fats and in many biologically important molecules.
Esters are among the most widespread of all naturally occurring compounds. Many simple esters are pleasant-smelling liquids that are responsible for the fragrant odors of fruits and flowers. For example, methyl butanoate is found in pineapple oil, and isopentyl acetate is a constituent of banana oil. The ester linkage is also present in animal fats and in many biologically important molecules.
 The chemical industry uses esters for a variety of purposes. Ethyl acetate, for instance, is a commonly used solvent, and dialkyl phthalates are used as plasticizers to keep polymers from becoming brittle. You may be aware that there is current concern about the possible toxicity of phthalates at high concentrations, although a recent assessment by the U.S. Food and Drug Administration found the risk to be minimal for most people, with the possible exception of male infants.
The chemical industry uses esters for a variety of purposes. Ethyl acetate, for instance, is a commonly used solvent, and dialkyl phthalates are used as plasticizers to keep polymers from becoming brittle. You may be aware that there is current concern about the possible toxicity of phthalates at high concentrations, although a recent assessment by the U.S. Food and Drug Administration found the risk to be minimal for most people, with the possible exception of male infants.
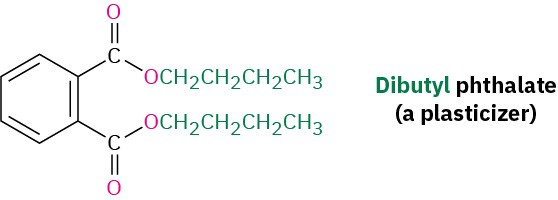 Amides, like esters, are abundant in all living organisms. Proteins, nucleic acids, and many pharmaceutical agents have amide functional groups. The reason for this abundance of amides is that they are stable in the aqueous conditions found in living organisms. Amides are the least reactive of the common acid derivatives and undergo relatively few nucleophilic acyl substitution reactions.
Amides, like esters, are abundant in all living organisms. Proteins, nucleic acids, and many pharmaceutical agents have amide functional groups. The reason for this abundance of amides is that they are stable in the aqueous conditions found in living organisms. Amides are the least reactive of the common acid derivatives and undergo relatively few nucleophilic acyl substitution reactions.