8.1 Structure and Stability of Benzene
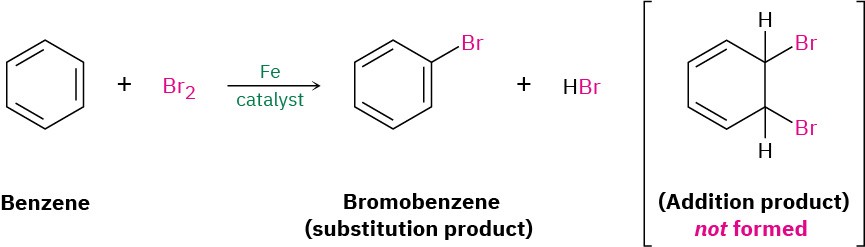
Benzene (C6H6) has six fewer hydrogens than the six-carbon cycloalkane cyclohexane (C6H12) and is clearly unsaturated, usually being represented as a six-membered ring with alternating double and single bonds. Yet it has been known since the mid-1800s that benzene is much less reactive than typical alkenes and fails to undergo typical alkene addition reactions. Cyclohexene, for instance, reacts rapidly with Br2 and gives the addition product 1,2-dibromocyclohexane, but benzene reacts slowly with Br2 and gives the substitution product C6H5Br rather than the addition product.
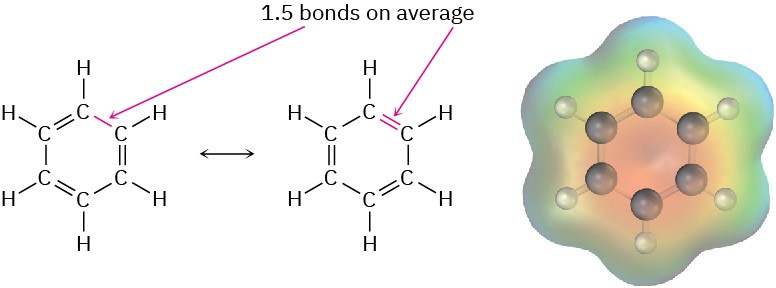
 Further evidence for the unusual nature of benzene is that all its carbon–carbon bonds have the same length—139 pm, which is intermediate between typical single (154 pm) and double (134 pm) bonds. In addition, an electrostatic potential map shows that the electron density in all six C–C bonds is identical. Thus, benzene is a planar molecule with the shape of a regular hexagon. All C–C–C bond angles are 120°, all six carbon atoms are sp2-hybridized, and each carbon has a p orbital perpendicular to the plane of the six-membered ring.
Further evidence for the unusual nature of benzene is that all its carbon–carbon bonds have the same length—139 pm, which is intermediate between typical single (154 pm) and double (134 pm) bonds. In addition, an electrostatic potential map shows that the electron density in all six C–C bonds is identical. Thus, benzene is a planar molecule with the shape of a regular hexagon. All C–C–C bond angles are 120°, all six carbon atoms are sp2-hybridized, and each carbon has a p orbital perpendicular to the plane of the six-membered ring.
 Because all six carbon atoms and all six p orbitals in benzene are equivalent, it’s impossible to define three localized π bonds in which a given p orbital overlaps only one neighboring p orbital. Rather, each p orbital overlaps equally well with both neighboring p orbitals, leading to a picture of benzene in which all six π electrons are free to move about the entire ring (Figure 8.2b). In resonance terms (Section 1.15 and Section 1.16), benzene is a hybrid of two equivalent forms. Neither form is correct by itself; the true structure of benzene is somewhere in between the two resonance forms but is impossible to draw with our usual conventions. Because of this resonance, benzene is more stable and less reactive than a typical alkene.
Because all six carbon atoms and all six p orbitals in benzene are equivalent, it’s impossible to define three localized π bonds in which a given p orbital overlaps only one neighboring p orbital. Rather, each p orbital overlaps equally well with both neighboring p orbitals, leading to a picture of benzene in which all six π electrons are free to move about the entire ring (Figure 8.2b). In resonance terms (Section 1.15 and Section 1.16), benzene is a hybrid of two equivalent forms. Neither form is correct by itself; the true structure of benzene is somewhere in between the two resonance forms but is impossible to draw with our usual conventions. Because of this resonance, benzene is more stable and less reactive than a typical alkene.
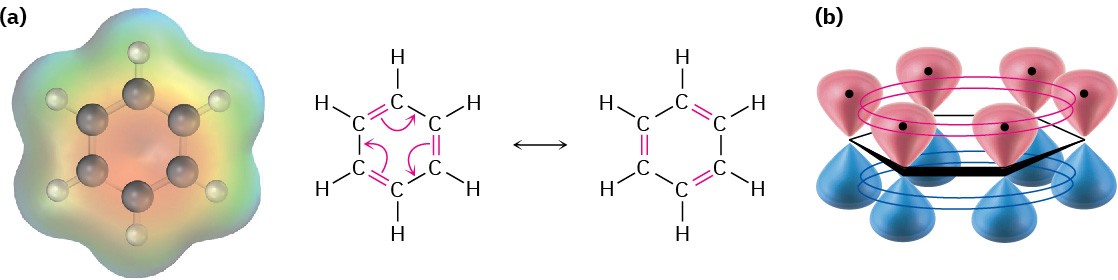 Figure 8.2 (a) An electrostatic potential map of benzene and (b) an orbital picture. Each of the six carbon atoms has a p orbital that can overlap equally well with neighboring p orbitals on both sides. As a result, all C–C bonds are equivalent and benzene must be represented as a hybrid of two resonance forms.
Figure 8.2 (a) An electrostatic potential map of benzene and (b) an orbital picture. Each of the six carbon atoms has a p orbital that can overlap equally well with neighboring p orbitals on both sides. As a result, all C–C bonds are equivalent and benzene must be represented as a hybrid of two resonance forms.
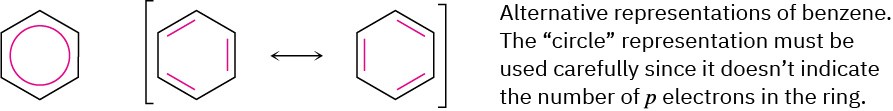
Chemists sometimes represent the two benzene resonance forms by using a circle to indicate the equivalence of the carbon–carbon bonds, but this representation has to be used carefully because it doesn’t indicate the number of π electrons in the ring. (How many electrons does a circle represent?) In this book, benzene and other aromatic compounds will be represented by single line-bond structures. We’ll be able to keep count of π electrons this way but must be aware of the limitations of the drawings.
 Problem 8.1
Problem 8.1
Pyridine is a flat, hexagonal molecule with bond angles of 120°. It undergoes substitution rather than addition and generally behaves like benzene. Draw a picture of the π orbitals of pyridine to explain its properties. Check your answer by looking ahead to Section 8.9.