7.1 The Discovery of Nucleophilic Substitution Reactions
Discovery of the nucleophilic substitution reaction of alkyl halides dates back to work carried out by the German chemist Paul Walden in 1896. Walden found that the pure enantiomeric (+)- and (–)-malic acids could be interconverted through a series of simple substitution reactions. When Walden treated (–)-malic acid with PCl5, he isolated (+)- chlorosuccinic acid. This, on treatment with wet Ag2O, gave (+)-malic acid. Similarly, reaction of (+)-malic acid with PCl5 gave (–)-chlorosuccinic acid, which was converted into (–)-malic acid when treated with wet Ag2O. The full cycle of reactions is shown in Figure 7.2.
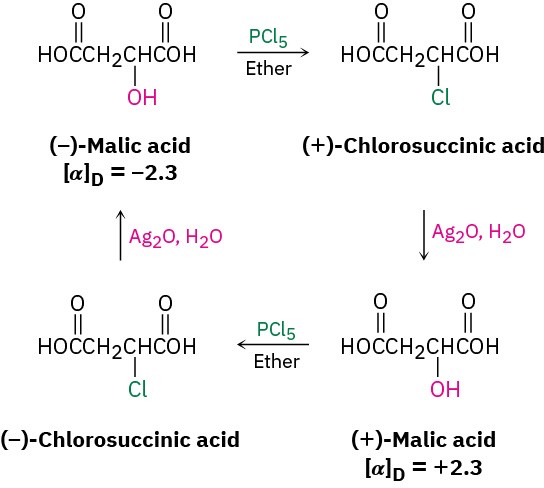
Figure 7.2 Walden’s cycle of reactions interconverting (+)- and (–)-malic acids.
At the time, the results were astonishing. The eminent chemist Emil Fischer called Walden’s discovery “the most remarkable observation made in the field of optical activity since the fundamental observations of Pasteur.” Because (–)-malic acid was converted into (+)-malic acid, some reactions in the cycle must have occurred with a change, or inversion, of configuration at the chirality center. But which ones, and how? (Remember from Section 3.5 that the direction of light rotation and the configuration of a chirality center aren’t directly related. You can’t tell by looking at the sign of rotation whether a change in configuration has occurred during a reaction.)
Today, we refer to the transformations taking place in Walden’s cycle as nucleophilic substitution reactions because each step involves the substitution of one nucleophile (chloride ion, Cl–, or hydroxide ion, HO–) by another. Nucleophilic substitution reactions are one of the most common and versatile reaction types in organic chemistry.
![]()
Worked Example 7.1: Predicting the Stereochemistry of a Nucleophilic Substitution Reaction
What product would you expect from a nucleophilic substitution reaction of (R)-1-bromo-1-phenylethane with cyanide ion, –C≡N, as nucleophile? Show the stereochemistry of both reactant and product, assuming that inversion of configuration occurs.
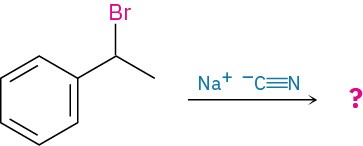
Strategy:
Draw the R enantiomer of the reactant, and then change the configuration of the chirality center while replacing the -Br with a -CN.
Solution:
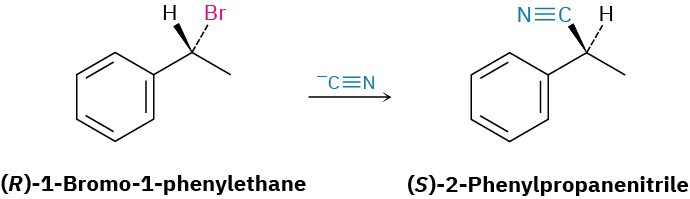
Problem 7.1
What product would you expect from a nucleophilic substitution reaction of (S)-2-bromohexane with acetate ion, CH3CO2–? Assume that inversion of configuration occurs, and show the stereochemistry of both the reactant and product.

