3 Why This Chapter?

Figure 3.1 Like the mountain whose image is reflected in a lake, many organic molecules also have mirror-image counterparts. (credit: modification of work “Crystal Lake sunrise reflection” by Sandy Horvath-Dori/Wikimedia Commons, CC BY 2.0)
Understanding the causes and consequences of molecular handedness is crucial to understanding organic and biological chemistry. The subject can be a bit complex at first, but the material covered in this chapter nevertheless forms the basis for much of the remainder of the book.
Are you right-handed or left-handed? You may not spend much time thinking about it, but handedness plays a surprisingly large role in your daily activities. Many musical instruments, such as oboes and clarinets, have a handedness to them; the last available softball glove always fits the wrong hand. The reason for these difficulties is that our hands aren’t identical; rather, they’re mirror images. When you hold a left hand up to a mirror, the image you see looks like a right hand. Try it.
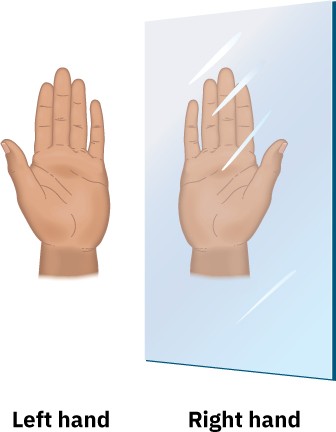 Handedness is also important in organic and biological chemistry, where it arises primarily as a consequence of the tetrahedral stereochemistry of sp3-hybridized carbon atoms. Many drugs and almost all the molecules in our bodies—amino acids, carbohydrates, nucleic acids, and many more—have a handedness. Furthermore, molecular handedness enables the precise interactions between enzymes and their substrates that are involved in the hundreds of thousands of chemical reactions on which life is based.
Handedness is also important in organic and biological chemistry, where it arises primarily as a consequence of the tetrahedral stereochemistry of sp3-hybridized carbon atoms. Many drugs and almost all the molecules in our bodies—amino acids, carbohydrates, nucleic acids, and many more—have a handedness. Furthermore, molecular handedness enables the precise interactions between enzymes and their substrates that are involved in the hundreds of thousands of chemical reactions on which life is based.

