5 Why This Chapter?
Figure 5.1 The Spectra fiber used to make the bulletproof vests used by police and military is made of ultra-high-molecular-weight polyethylene, a simple alkene polymer. (credit: modification of work “US Navy 081028-N-3857R-007 Seabees participate in a chemical, biological and radiological warfare drill” by U.S. Navy photo by Mass Communication Specialist 1st Class Chad Runge/Wikimedia Commons, Public Domain)
Much of the background needed to understand organic reactions has now been covered, and it’s time to begin a systematic description of the major functional groups. In this chapter on alkenes, and in future chapters on other functional groups, we’ll discuss a variety of reactions, but try to focus on the general principles and patterns of reactivity that tie organic chemistry together. There are no shortcuts; you have to know the reactions to understand organic and biological chemistry.
Alkene addition reactions occur widely, both in the laboratory and in living organisms. Although we’ve studied only the addition of HX thus far, many closely related reactions also take place. In this chapter, we’ll see briefly how alkenes are prepared and we’ll discuss further examples of alkene addition reactions. Particularly important are the addition of a halogen (X2) to give a 1,2-dihalide, addition of a hypohalous acid (HOX) to give a halohydrin, addition of water to give an alcohol, addition of hydrogen to give an alkane, addition of a single oxygen to give a three-membered cyclic ether called an epoxide, and addition of two hydroxyl groups to give a 1,2-diol.
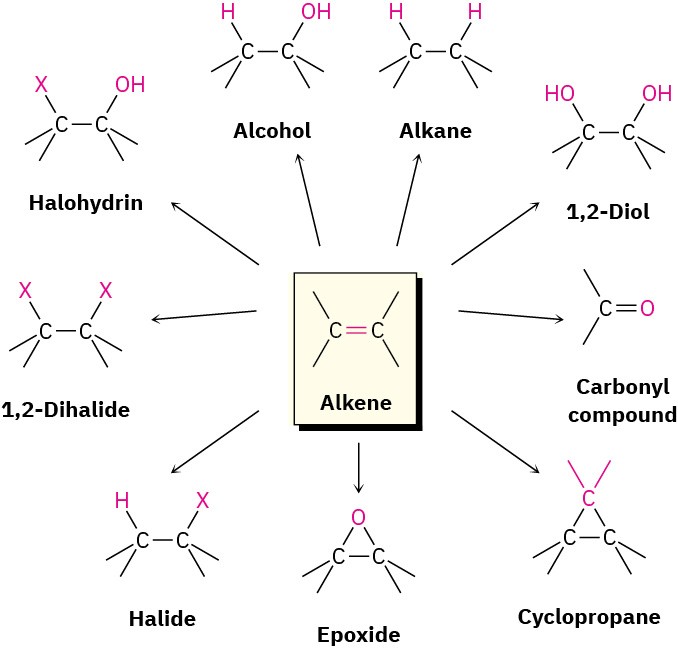 Figure 5.2 Some useful alkene reactions.
Figure 5.2 Some useful alkene reactions.
Alkynes are less common than alkenes, both in the laboratory and in living organisms, so we won’t cover them in great detail. The real importance of this chapter is that we’ll use alkyne chemistry as a vehicle to begin looking at some of the general strategies used in organic synthesis—the construction of complex molecules in the laboratory. Without the ability to design and synthesize new molecules in the laboratory, many of the medicines we take for granted would not exist and few new ones would be made.
An alkyne is a hydrocarbon that contains a carbon–carbon triple bond. Acetylene, H–C≡C–H, the simplest alkyne, was once widely used in industry as a starting material for the preparation of acetaldehyde, acetic acid, vinyl chloride, and other high-volume chemicals, but more efficient routes to these substances using ethylene as starting material are now available. Acetylene is still used in the preparation of acrylic polymers, such as Plexiglas and Lucite, but is probably best known as the gas burned in high-temperature oxy–acetylene welding torches.
In addition to simple alkynes with one triple bond, research is also being carried out on polyynes—linear carbon chains of alternating single and triple bonds. Polyynes with up to eight triple bonds are thought to be present in interstellar space, and evidence has been presented for the existence of carbyne, an allotrope of carbon consisting of alternating single and triple bonds in long chains of indefinite length. The electronic properties of polyynes are being explored for potential use in nanotechnology applications.