9 Why This Chapter?

Figure 9.1 The phenol resveratrol, found in the skin of red grapes, continues to be studied for its potential anti-cancer, antiarthritic, and hypoglycemic properties. (credit: modification of work “Weinreben-Ötlingen” by Pierre Likissas/Wikimedia Commons, CC BY 3.0)
Up to this point, we’ve focused on developing some general ideas of organic reactivity, looking at the chemistry of hydrocarbons and alkyl halides, and examining some of the tools used in structural studies. With that background, it’s now time to begin a study of the oxygen-containing functional groups that lie at the heart of organic and biological chemistry.
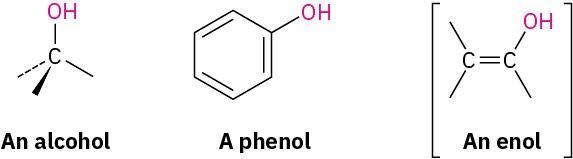
Alcohols and phenols can be thought of as organic derivatives of water in which one of water’s hydrogens is replaced by an organic group: H–O–H versus R–O–H and Ar–O–H. In practice, the name alcohol is restricted to compounds that have their –OH group bonded to a saturated, sp3-hybridized carbon atom, while compounds with their –OH group bonded to a vinylic, sp2-hybridized carbon are called enols.
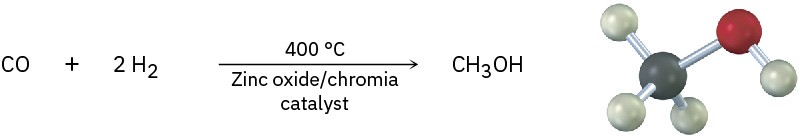
 Alcohols occur widely in nature and have many industrial and pharmaceutical applications. Methanol, for instance, is one of the most important of all industrial chemicals. Historically, methanol was prepared by heating wood in the absence of air and thus came to be called wood alcohol. Today, approximately 173 million tons (50 billion gallons) of methanol is manufactured worldwide each year, most of it by catalytic reduction of carbon monoxide with hydrogen gas. Methanol is toxic to humans, causing blindness in small doses (15 mL) and death in larger amounts (100–250 mL). Industrially, it is used both as a solvent and as a starting material for production of formaldehyde (CH2O) and acetic acid (CH3CO2H).
Alcohols occur widely in nature and have many industrial and pharmaceutical applications. Methanol, for instance, is one of the most important of all industrial chemicals. Historically, methanol was prepared by heating wood in the absence of air and thus came to be called wood alcohol. Today, approximately 173 million tons (50 billion gallons) of methanol is manufactured worldwide each year, most of it by catalytic reduction of carbon monoxide with hydrogen gas. Methanol is toxic to humans, causing blindness in small doses (15 mL) and death in larger amounts (100–250 mL). Industrially, it is used both as a solvent and as a starting material for production of formaldehyde (CH2O) and acetic acid (CH3CO2H).
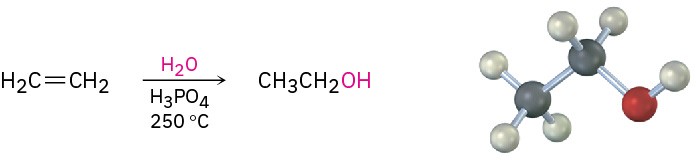
 Ethanol was one of the first organic chemicals to be prepared and purified. Its production by fermentation of grains and sugars has been carried out for perhaps 9000 years, and its purification by distillation goes back at least as far as the 12th century. Today, approximately 88 million tons (26 billion gallons) of ethanol are produced worldwide each year, most of it by fermentation of corn, barley, sorghum, and other plant sources. Almost all of this ethanol is used for bus and automobile fuel.
Ethanol was one of the first organic chemicals to be prepared and purified. Its production by fermentation of grains and sugars has been carried out for perhaps 9000 years, and its purification by distillation goes back at least as far as the 12th century. Today, approximately 88 million tons (26 billion gallons) of ethanol are produced worldwide each year, most of it by fermentation of corn, barley, sorghum, and other plant sources. Almost all of this ethanol is used for bus and automobile fuel.
Ethanol for industrial use as a solvent or chemical intermediate is largely obtained by acid-catalyzed hydration of ethylene at high temperature.
 Phenols occur widely throughout nature and also serve as intermediates in the industrial synthesis of products as diverse as adhesives and antiseptics. Phenol itself is a general disinfectant found in coal tar; methyl salicylate is a flavoring agent found in oil of wintergreen; and urushiols are the allergenic constituents of poison oak and poison ivy. Note that the word phenol is the name both of the specific compound (hydroxybenzene) and of the class of compounds.
Phenols occur widely throughout nature and also serve as intermediates in the industrial synthesis of products as diverse as adhesives and antiseptics. Phenol itself is a general disinfectant found in coal tar; methyl salicylate is a flavoring agent found in oil of wintergreen; and urushiols are the allergenic constituents of poison oak and poison ivy. Note that the word phenol is the name both of the specific compound (hydroxybenzene) and of the class of compounds.
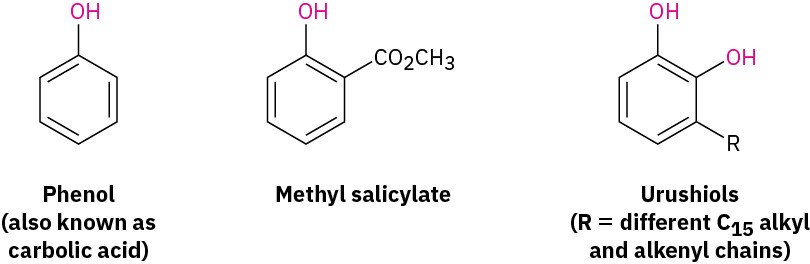

Figure 9.2 The appalling and unforgettable odor of skunks is due to a mixture of several simple thiols. (credit: modification of work “Striped Skunk” by Tom Friedel/Wikimedia Commons, CC BY 3.0)
This chapter also finishes the coverage of functional groups with C–O and C–S single bonds. We’ll focus primarily on ethers and take only a brief look at thiols and sulfides before going on to an extensive coverage of compounds with C=O double bonds in Chapters 10 through 11.
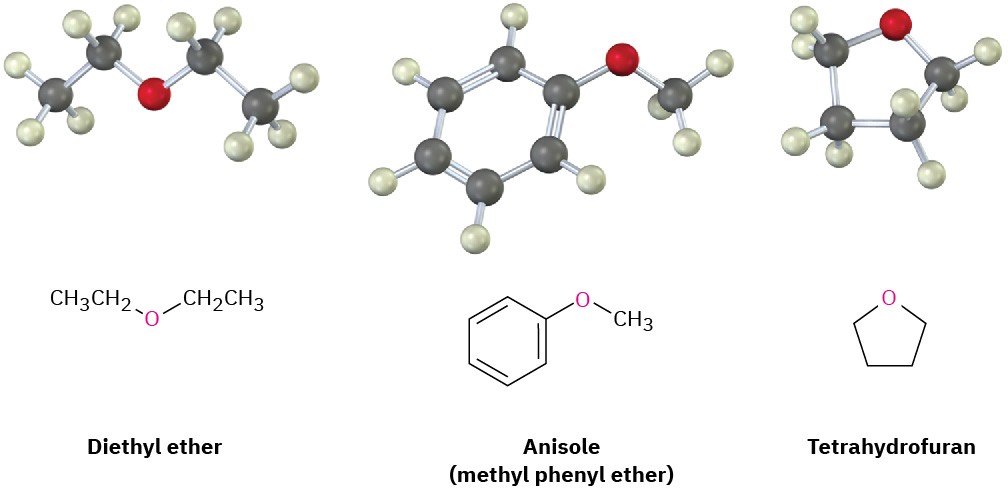
Ethers (R–O–R′), like the alcohols, are organic derivatives of water, but they have two organic groups bonded to the same oxygen atom rather than one. The organic groups might be alkyl, aryl, or vinylic, and the oxygen atom might be in an open chain or a ring.
Perhaps the most well-known ether is diethyl ether, which has a long history of medicinal use as an anesthetic and industrial use as a solvent. Other useful ethers include anisole, a pleasant-smelling aromatic ether used in perfumery, and tetrahydrofuran (THF), a cyclic ether often used as a solvent.
 Thiols (R–S–H) and sulfides (R–S–R′) are sulfur analogs of alcohols and ethers, respectively. Both functional groups are found in various biomolecules, although not as commonly as their oxygen-containing relatives.
Thiols (R–S–H) and sulfides (R–S–R′) are sulfur analogs of alcohols and ethers, respectively. Both functional groups are found in various biomolecules, although not as commonly as their oxygen-containing relatives.

