Module 6: Chain Growth Synthesis
6.0 Introduction & Study Guide
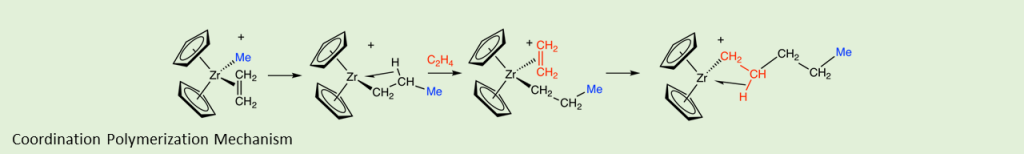 We will cover the basics of chain growth polymerizations. You are responsible for learning the overall synthesis reactions. We will also cover the mechanisms of chain growth polymerizations. You will not be expected to draw out complete mechanisms, but you will need to recognize a reaction as anionic (or cationic or free radical or coordination) and whether the reaction is showing initiation, propagation or termination. We will cover the importance of coordination polymerization and living polymerizations.
We will cover the basics of chain growth polymerizations. You are responsible for learning the overall synthesis reactions. We will also cover the mechanisms of chain growth polymerizations. You will not be expected to draw out complete mechanisms, but you will need to recognize a reaction as anionic (or cationic or free radical or coordination) and whether the reaction is showing initiation, propagation or termination. We will cover the importance of coordination polymerization and living polymerizations.
Read Sections 3.3 – 3.5: the sections on Chain Growth Polymerizations in Chapter 3: Polymer Synthesis of the Polymer Science and Engineering eBook (or the equivalent in a print version). Section 3.6: Polymerization Processes is optional, but interesting!
Study Guide for Module 6: Chain Growth Synthesis
By the end of the module, can you do the following?
- Given a chain growth polymer structure, determine what monomer or monomers it may have been made from.
- Complete polymerization reactions for chain growth monomers; mechanisms are not required.
- Given part of a chain growth reaction scheme, identify whether the reaction is initiation, propagation, or termination and what type of polymerization it is (free radical, anionic, cationic, coordination).
- Choose the best type of chain growth polymerization (free radical, anionic, cationic, coordination) for a given monomer or desired polymer characteristics. For example, if you want isotactic polypropylene, you would choose coordination polymerization.
- Explain what a living polymerization is and why it is important.
Get your own copy of the Module 06 Study Guide and Checklist (link goes to a “force copy” Google Doc).
