18 Ecosystem Ecology
At the end of this chapter you will be able to:
- Differentiate between food chains and food webs and recognize the importance of each
- Describe how organisms acquire energy in a food web and why this energy decreases from one trophic level to the next
- Explain how the efficiency of energy transfers between trophic levels affects ecosystems
- Discuss the biogeochemical cycles of carbon, nitrogen, and phosphorus
- Explain how human activities have impacted these cycles and the resulting potential consequences
Introduction
An ecosystem is really a view of communities from a perspective of how energy is moving through the system and how nutrients are cycling through the system. Ecosystems can be small, such as the tide pools found near the rocky shores of many oceans, or large, such as those found in the tropical rainforest of the Amazon in Brazil (Fig 1)
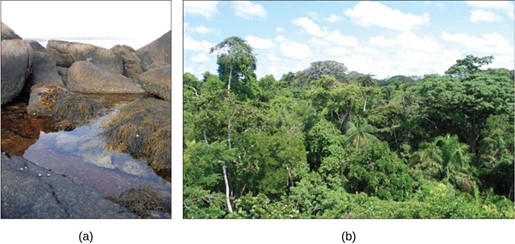
Figure 1: A (a) tidal pool ecosystem in Matinicus Island, Maine, is a small ecosystem, while the (b) Amazon rainforest in Brazil is a large ecosystem. (credit a: modification of work by Jim Kuhn; credit b: modification of work by Ivan Mlinaric)
Life in an ecosystem often involves competition for limited resources, which occurs both within a single species and between different species. Organisms compete for food, water, sunlight, space, and mineral nutrients. These resources provide the energy for metabolic processes and the matter to make up organisms’ physical structures. Other critical factors influencing community dynamics are the components of its physical environment: a habitat’s climate (seasons, sunlight, and rainfall), elevation, and geology. These can all be important environmental variables that determine which organisms can exist within a particular area.
Freshwater ecosystems are the least common, occurring on only 1.8 percent of Earth’s surface. These systems comprise lakes, rivers, streams, and springs; they are quite diverse, and support a variety of animals, plants, fungi, protists and prokaryotes.
Marine ecosystems are the most common, comprising 75 percent of Earth’s surface and consisting of three basic types: shallow ocean, deep ocean water, and deep ocean bottom. Shallow ocean ecosystems include extremely biodiverse coral reef ecosystems, yet the deep ocean water is known for large numbers of plankton and krill (small crustaceans) that support it. These two environments are especially important to aerobic respirators worldwide, as the phytoplankton perform 40 percent of all photosynthesis on Earth. Although not as diverse as the other two, deep ocean bottom ecosystems contain a wide variety of marine organisms. Such ecosystems exist even at depths where light is unable to penetrate through the water.
Terrestrial ecosystems, also known for their diversity, are grouped into large categories called biomes. A biome is a large-scale community of organisms, primarily defined on land by the dominant plant types that exist in geographic regions of the planet with similar climatic conditions. Examples of biomes include tropical rainforests, savannas, deserts, grasslands, temperate forests, and tundras. Grouping these ecosystems into just a few biome categories obscures the great diversity of the individual ecosystems within them. For example, the saguaro cacti (Carnegiea gigantean) and other plant life in the Sonoran Desert, in the United States, are relatively diverse compared with the desolate rocky desert of Boa Vista, an island off the coast of Western Africa.
Food Chains and Food Webs
A food chain is a linear sequence of organisms through which nutrients and energy pass as one organism eats another; the levels in the food chain are producers, primary consumers, higher-level consumers, and finally decomposers. These levels are used to describe ecosystem structure and dynamics. There is a single path through a food chain. Each organism in a food chain occupies a specific trophic level (energy level), its position in the food chain or food web.
In many ecosystems, the base, or foundation, of the food chain consists of photosynthetic organisms (plants or phytoplankton), which are called producers. The organisms that consume the producers are herbivores: the primary consumers. Secondary consumers are usually carnivores that eat the primary consumers. Tertiary consumers are carnivores that eat other carnivores. Higher-level consumers feed on the next lower trophic levels, and so on, up to the organisms at the top of the food chain: the apex consumers. In the Lake Ontario food chain (Fig 2), the Chinook salmon is the apex consumer at the top of this food chain.
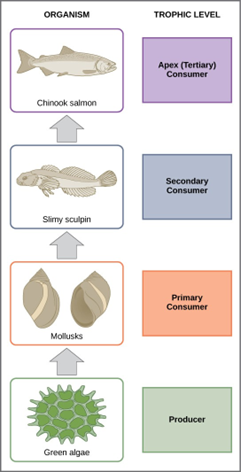
Figure 2: These are the trophic levels of a food chain in Lake Ontario at the United States–Canada border. Energy and nutrients flow from photosynthetic green algae at the base to the top of the food chain: the Chinook salmon. (credit: modification of work by National Oceanic and Atmospheric Administration/NOAA)
One major factor that limits the number of steps in a food chain is energy. Energy is lost at each trophic level and between trophic levels as heat and in the transfer to decomposers (Fig 3). Thus, after a limited number of trophic energy transfers, the amount of energy remaining in the food chain may not be great enough to support viable populations at yet a higher trophic level.
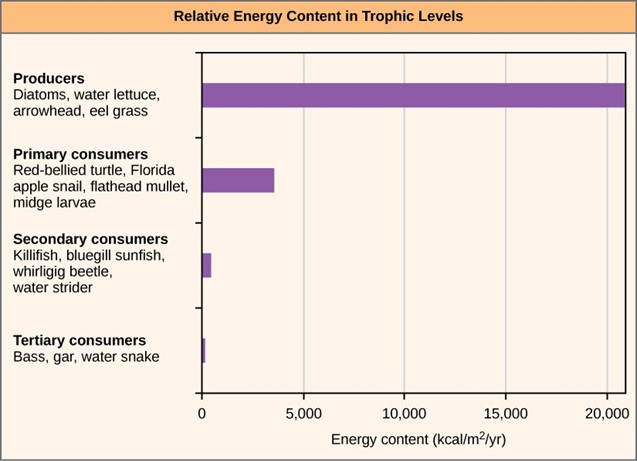
Figure 3: The relative energy in trophic levels in a Silver Springs, Florida, ecosystem is shown. Each trophic level has less energy available, and usually, but not always, supports a smaller mass of organisms at the next level.
In general, the amount of energy that is available to the next trophic level is only about 10% of what was available. That means that at the first trophic level, autotrophs are using ~90% of the energy that they fix during photosynthesis. They are using the energy to grow taller, grow more roots, produce flowers, and engage in metabolic processes. During such conversions, some energy may be lost as heat. When a primary consumer eats the plants, they will not be able to successfully assimilate all the energy as well. This is why the amount of energy decreases so quickly as you move up the trophic levels and why you will only find typically 4 or 5 trophic levels in any one ecosystem or community.
This concept is called the 10% rule and is a fundamental concept in ecosystem ecology and suggests that, on average, only about 10% of the energy available at one trophic level is transferred to the next trophic level. When scientists studied energy transfer they learned that some ecosystems might pass more than 10% in some cases and less than 10% in other cases, but on average, they found 10% was found. That is, On average, only approximately 10% of the energy from one trophic level is converted into biomass and becomes available as food for the next trophic level. The rest of the energy is lost as heat during metabolic processes or is used for the organism’s own growth and maintenance.
The 10% rule has important implications for the structure of ecosystems. It explains why ecosystems typically a pyramid-shaped structure with a broad base of primary producers supporting progressively smaller populations of herbivores, carnivores, and so on (Fig 4). The pyramid can be used not only to show energy availability, but also biomass and numbers or individuals. Notice in each case that the amount of biomass or individuals decrease as you move to a higher trophic level. This is again becuase there is not enough energy to support more biomass or individuals. Ecologists can refer to this as bottom-up control of an ecosystem. The bottom trophic level dictates the community structure above it based on how much energy it can fix in photosynthesis.
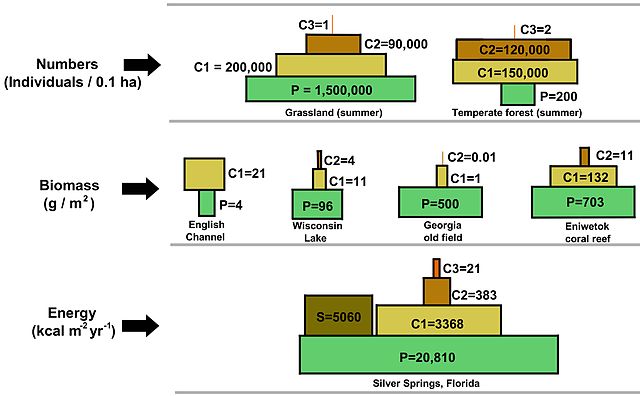
Figure 4: Three different kinds of trophic pyramids are illustrated, including a pyramid of numbers (top), pyramid of biomass (middle), and pyramid of energy). The illustration is not drawn to scale and is redrawn from figure 3-12 in (2005) Fundamentals of ecology, Brooks Cole, pp. 598,which is based on published experimental data. Notation: P=Producer, C1=primary consumer, C2=secondary consumer, C3=tertiary consumer, S=saprotroph. From Thompsma, CC, via Wikimedia Commons
There is one problem when using food chains to describe most ecosystems. Even when all organisms are grouped into appropriate trophic levels, some of these organisms can feed on more than one trophic level; likewise, some of these organisms can also be fed on from multiple trophic levels. In addition, species feed on and are eaten by more than one species. In other words, the linear model of ecosystems, the food chain, is a hypothetical, overly simplistic representation of ecosystem structure. A holistic model—which includes all the interactions between different species and their complex interconnected relationships with each other and with the environment—is a more accurate and descriptive model for ecosystems. A food web is a concept that accounts for the multiple trophic (feeding) interactions between each species and the many species it may feed on, or that feed on it. In a food web, the several trophic connections between each species and the other species that interact with it may cross multiple trophic levels. The matter and energy movements of virtually all ecosystems are more accurately described by food webs (Fig 5).
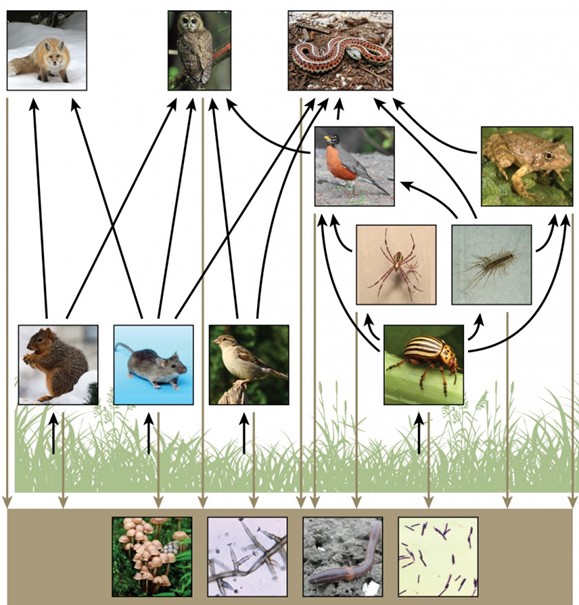
Figure 5: This food web shows the interactions between organisms across trophic levels. Arrows point from an organism that is consumed to the organism that consumes it. All the producers and consumers eventually become nourishment for the decomposers (fungi, mold, earthworms, and bacteria in the soil). (credit “fox”: modification of work by Kevin Bacher, NPS; credit “owl”: modification of work by John and Karen Hollingsworth, USFWS; credit “snake”: modification of work by Steve Jurvetson; credit “robin”: modification of work by Alan Vernon; credit “frog”: modification of work by Alessandro Catenazzi; credit “spider”: modification of work by “Sanba38″/Wikimedia Commons; credit “centipede”: modification of work by “Bauerph”/Wikimedia Commons; credit “squirrel”: modification of work by Dawn Huczek; credit “mouse”: modification of work by NIGMS, NIH; credit “sparrow”: modification of work by David Friel; credit “beetle”: modification of work by Scott Bauer, USDA Agricultural Research Service; credit “mushrooms”: modification of work by Chris Wee; credit “mold”: modification of work by Dr. Lucille Georg, CDC; credit “earthworm”: modification of work by Rob Hille; credit “bacteria”: modification of work by Don Stalons, CDC)
Two general types of food webs are often shown interacting within a single ecosystem. A grazing food web has plants or other photosynthetic organisms at its base, followed by herbivores and various carnivores. A detrital food web consists of a base of organisms that feed on decaying organic matter (dead organisms), including decomposers (which break down dead and decaying organisms) and detritivores (which consume organic detritus). These organisms are usually bacteria, fungi, and invertebrate animals that recycle organic material back into the biotic part of the ecosystem as they themselves are consumed by other organisms. As ecosystems require a method to recycle material from dead organisms, grazing food webs have an associated detrital food web. For example, in a meadow ecosystem, plants may support a grazing food web of different organisms, primary and other levels of consumers, while at the same time supporting a detrital food web of bacteria and fungi feeding off dead plants and animals. Simultaneously, a detrital food web can contribute energy to a grazing food web, as when a robin eats an earthworm.
It is also worth noting that you might also find top-down control in an ecosystem. With top-down control, the predators/top carnivores are controlling things. Their numbers and behavior dictate how many prey are found, which in turn dictates how many plants and autotrophs are consumed. In this way, in ecosystems, top predators, often at the highest trophic level, play a critical role in regulating the populations of species at lower trophic levels. They do so by controlling the abundance of herbivores or lower-level consumers. When top predators are present and exert their influence, they can limit the populations of herbivores, which in turn can lead to changes in the abundance of primary producers (plants) that are the primary food source for herbivores. These effects can cascade through the ecosystem, affecting multiple trophic levels. In some ecosystems, these top predators control the herbivore populations so that the herbivore populations do not explode, which could lead to overgrazing of plant communities, which can have detrimental effects on ecosystem structure and diversity.
A classic example of top-down control theory is the reintroduction of wolves into Yellowstone National Park in the United States. With the return of wolves, the populations of elk, which were overgrazing and affecting plant communities, were controlled. This had cascading effects, leading to a more diverse and balanced ecosystem.
How Organisms Acquire Energy in Food Webs
All living things require energy in one form or another. Energy is used by most complex metabolic pathways (usually in the form of ATP), especially those responsible for building large molecules from smaller compounds. Living organisms would not be able to assemble macromolecules (proteins, lipids, nucleic acids, and complex carbohydrates) from their monomers without a constant energy input.
Food-web diagrams illustrate how energy flows directionally through ecosystems. They can also indicate how efficiently organisms acquire energy, use it, and how much remains for use by other organisms of the food web. Energy is acquired by living things in two ways: autotrophs harness light or chemical energy and heterotrophs acquire energy through the consumption and digestion of other living or previously living organisms.
Photosynthetic and chemosynthetic organisms are autotrophs, which are organisms capable of synthesizing their own food (more specifically, capable of using inorganic carbon as a carbon source). Photosynthetic autotrophs (photoautotrophs) use sunlight as an energy source, and chemosynthetic autotrophs (chemoautotrophs) use inorganic molecules as an energy source. Autotrophs are critical for most ecosystems: they are the producer trophic level. Without these organisms, energy would not be available to other living organisms, and life itself would not be possible.
Photoautotrophs, such as plants, algae, and photosynthetic bacteria, are the energy source for a majority of the world’s ecosystems. These ecosystems are often described by grazing and detrital food webs. Photoautotrophs harness the Sun’s solar energy by converting it to chemical energy in the form of ATP (and NADP). The energy stored in ATP is used to synthesize complex organic molecules, such as glucose. The rate at which photosynthetic producers incorporate energy from the Sun is called gross primary productivity. However, not all of the energy incorporated by producers is available to the other organisms in the food web because producers must also grow and reproduce, which consumes energy. Net primary productivity is the energy that remains in the producers after accounting for these organisms’ respiration and heat loss. The net productivity is then available to the primary consumers at the next trophic level.
Chemoautotrophs are primarily bacteria and archaea that are found in rare ecosystems where sunlight is not available, such as those associated with dark caves or hydrothermal vents at the bottom of the ocean (Fig 6). Many chemoautotrophs in hydrothermal vents use hydrogen sulfide (H2S), which is released from the vents as a source of chemical energy; this allows them to synthesize complex organic molecules, such as glucose, for their own energy and, in turn, supplies energy to the rest of the ecosystem.

Figure 6: Photo of one of the largest concentrations of tubeworms, Riftia pachyptila, observed, with anemones and mussels colonizing in close proximity. Chemosynthetic bacteria, which are not observable, live inside the tube worms and provide the basis for life at these great depths. From the 2011 NOAA Galapagos Rift Expedition. The original NOAA image has been modified by increasing brightness.
Biological Amplification or Biomagnification
One of the most important consequences of ecosystem dynamics in terms of human impact is biomagnification. Biomagnification is the increasing concentration of persistent, toxic substances in organisms at each successive trophic level. These are substances that are fat soluble, not water soluble, and are stored in the fat reserves of each organism. Many substances have been shown to biomagnify, including classical studies with the pesticide dichlorodiphenyltrichloroethane (DDT), which were described in the 1960s bestseller, Silent Spring by Rachel Carson. DDT was a commonly used pesticide before its dangers to apex consumers, such as the bald eagle, became known. In aquatic ecosystems, organisms from each trophic level consumed many organisms in the lower level, which caused DDT to increase in birds (apex consumers) that ate fish. Thus, the birds accumulated sufficient amounts of DDT to cause fragility in their eggshells. This effect increased egg breakage during nesting and was shown to have devastating effects on these bird populations. The use of DDT was banned in the United States in the 1970s.
Other substances that biomagnify are polychlorinated biphenyls (PCB), which were used as coolant liquids in the United States until their use was banned in 1979, and heavy metals, such as mercury, lead, and cadmium. These substances are best studied in aquatic ecosystems, where predatory fish species accumulate very high concentrations of toxic substances that are at quite low concentrations in the environment and in producers. As illustrated in a study performed by the NOAA in the Saginaw Bay of Lake Huron of the North American Great Lakes (Fig 7), PCB concentrations increased from the producers of the ecosystem (phytoplankton) through the different trophic levels of fish species. The apex consumer, the walleye, has more than four times the amount of PCBs compared to phytoplankton. Also, based on results from other studies, birds that eat these fish may have PCB levels at least one order of magnitude higher than those found in the lake fish.
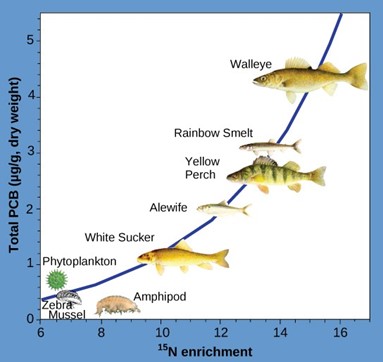
Figure 7: This graph shows the PCB concentrations found at the various trophic levels in the Saginaw Bay ecosystem of Lake Huron. Notice that the fish in the higher trophic levels accumulate more PCBs than those in lower trophic levels. (credit: Patricia Van Hoof, NOAA)
Other concerns have been raised by the biomagnification of heavy metals, such as mercury and cadmium, in certain types of seafood. The United States Environmental Protection Agency recommends that pregnant women and young children should not consume any swordfish, shark, king mackerel, or tilefish because of their high mercury content. These individuals are advised to eat fish low in mercury: salmon, shrimp, pollock, and catfish. Biomagnification is a good example of how ecosystem dynamics can affect our everyday lives, even influencing the food we eat.
Biogeochemical Cycles
Energy as discussed above flows from one trophic level to the next. On the other hand, nutrients, such as calcium and nitrogen, cycle through an ecosystem. Nutrients will move from abiotic components of the ecosystem, such as soil, water, or the atmosphere, to biotic components, such as bacteria, plants, and animals, and then cycle again.
The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath Earth’s surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in the cycling of elements on Earth. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their nonliving environment is called a biogeochemical cycle.
Carbon is found in all organic macromolecules and is an important constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is critical to human agriculture. Phosphorus, a major component of nucleic acids, is one of the main ingredients (along with nitrogen) in artificial fertilizers used in agriculture, which has environmental impacts on our surface water.
The Carbon Cycle
Carbon is the fourth most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain energy, and many of these compounds from plants and algae have remained stored as fossilized carbon, which humans use as fuel. Since the 1800s, the use of fossil fuels has accelerated. As global demand for Earth’s limited fossil fuel supplies has risen since the beginning of the Industrial Revolution, the amount of carbon dioxide in our atmosphere has increased as the fuels are burned. This increase in carbon dioxide has been associated with climate change and is a major environmental concern worldwide.
The carbon cycle is most easily studied as two interconnected subcycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes (Fig 8).
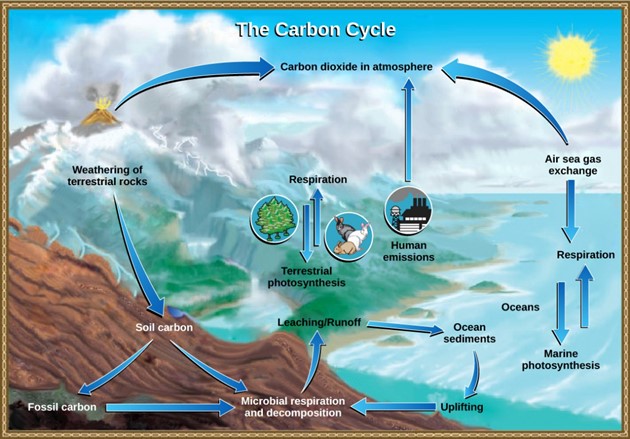
Figure 8: Carbon dioxide gas exists in the atmosphere and is dissolved in water. Photosynthesis converts carbon dioxide gas to organic carbon, and respiration cycles the organic carbon back into carbon dioxide gas. Long-term storage of organic carbon occurs when matter from living organisms is buried deep underground and becomes fossilized. Volcanic activity and, more recently, human emissions bring this stored carbon back into the carbon cycle. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
Living organisms are connected in many ways, even between ecosystems. A good example of this connection is the exchange of carbon between heterotrophs and autotrophs within and between ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that autotrophs use to build multi-carbon, high-energy compounds, such as glucose. The energy harnessed from the Sun is used by these organisms to form the covalent bonds that link carbon atoms together. These chemical bonds store this energy for later use in the process of respiration. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (carbonic acid, HCO3–). However the carbon dioxide is acquired, a byproduct of fixing carbon in organic compounds is oxygen. Photosynthetic organisms are responsible for maintaining approximately 21 percent of the oxygen content of the atmosphere that we observe today.
The partners in biological carbon exchange are the heterotrophs (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them and breaking them down by respiration to obtain cellular energy, such as ATP. The most efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Autotrophs also respire and consume the organic molecules they form: using oxygen and releasing carbon dioxide. They release more oxygen gas as a waste product of photosynthesis than they use for their own respiration; therefore, there is excess available for the respiration of other aerobic organisms. Gas exchange through the atmosphere and water is one way that the carbon cycle connects all living organisms on Earth.
The movement of carbon through land, water, and air is complex, and, in many cases, it occurs much more slowly geologically than the movement between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs, which include the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, rocks (including fossil fuels), and Earth’s interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide that is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each, and each one affects the other reciprocally. Carbon dioxide (CO2) from the atmosphere dissolves in water and, unlike oxygen and nitrogen gas, reacts with water molecules to form ionic compounds. Some of these ions combine with calcium ions in the seawater to form calcium carbonate (CaCO3), a major component of the shells of marine organisms. These organisms eventually form sediments on the ocean floor. Over geologic time, the calcium carbonate forms limestone, which comprises the largest carbon reservoir on Earth.
On land, carbon is stored in soil as organic carbon as a result of the decomposition of living organisms or from weathering of terrestrial rock and minerals. Deeper under the ground, at land and at sea, are fossil fuels, the anaerobically decomposed remains of plants that take millions of years to form. Fossil fuels are considered a non-renewable resource because their use far exceeds their rate of formation. A non-renewable resource is either regenerated very slowly or not at all. Another way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean floor are taken deep within Earth by the process of subduction: the movement of one tectonic plate beneath another. Carbon is released as carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.
Carbon dioxide is also added to the atmosphere by the animal husbandry practices of humans. The large number of land animals raised to feed Earth’s growing human population results in increased carbon-dioxide levels in the atmosphere caused by their respiration. This is another example of how human activity indirectly affects biogeochemical cycles in a significant way. Although much of the debate about the future effects of increasing atmospheric carbon on climate change focuses on fossils fuels, scientists take natural processes, such as volcanoes, plant growth, soil carbon levels, and respiration, into account as they model and predict the future impact of this increase.
Eutrophication
When excess nutrients, such as nitrogen, enter an aquatic ecosystem, a cascade of events occurs. For some species, these excess nutrients are a stimulant and cause a population explosion and for other species, the nutrients ultimately cause harm. Initially, there is a species whose population benefits from the nitrogen acting as a fertilizer. For example, a species of algae in the water will get a “jump start” with the nitrogen and it can photosynthesize rapidly, and its population can expand rapidly. As its population increases, it will spread and often times can physically be seen as a blanket of “green” on the surface. In a pond, this is described as “pond scum.” With this increase in numbers, the amount of sunlight that can penetrate into the water decreases. Any plants that are anchored and underneath the algae may struggle to survive. Regardless, when the algae die after living out their natural life span, they will fall to the bottom of the pond or lake and now decomposers, such as bacteria, will have an increase in their material to decompose. The bacteria decomposing all the dead algae will use a lot of oxygen in the process. Once this phase begins, the amount of oxygen in the water will decrease dramatically which can lead to fish kills (Fig 10).
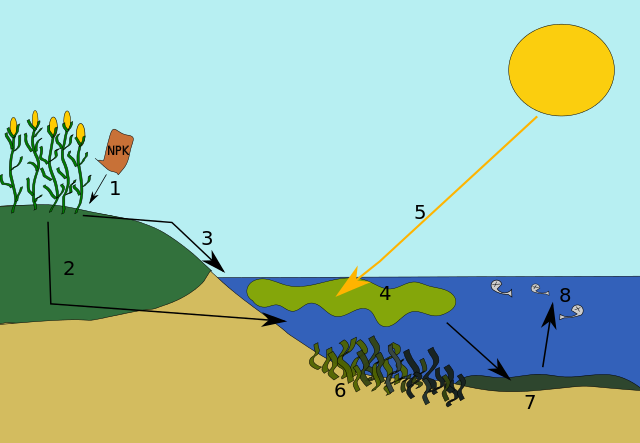
Figure 10: 1. Excess nutrients are applied to the soil. 2. Some nutrients leach into the soil where they can remain for years. Eventually, they get drained into the water body. 3. Some nutrients run off over the ground into the body of water. 4. The excess nutrients cause an algal bloom. 7. Eventually, the algal bloom dies and sinks to the bottom of the lake. Bacteria begins to decompose the remains, using up oxygen for respiration. 8. The decomposition causes the water to become depleted of oxygen. Larger life forms, such as fish, suffocate to death. This body of water can no longer support life. Kungfucrab, CC via Wikimedia Commons
The Nitrogen Cycle
Getting nitrogen into the living world is difficult. Plants and phytoplankton are not equipped to incorporate nitrogen from the atmosphere (which exists as tightly bonded, triple covalent N2) even though this molecule comprises approximately 78 percent of the atmosphere. Nitrogen enters the living world via free-living and symbiotic bacteria, which incorporate nitrogen into their macromolecules through nitrogen fixation (conversion of N2). Cyanobacteria live in most aquatic ecosystems where sunlight is present; they play a key role in nitrogen fixation. Cyanobacteria are able to use inorganic sources of nitrogen to “fix” nitrogen. Rhizobium bacteria live symbiotically in the root nodules of legumes (such as peas, beans, and peanuts) and provide them with the organic nitrogen they need. Free-living bacteria, such as Azotobacter, are also important nitrogen fixers.
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as primary production and decomposition, are limited by the available supply of nitrogen. The nitrogen that enters living systems by nitrogen fixation is eventually converted from organic nitrogen back into nitrogen gas by bacteria (Fig 11). This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (NH4+) by certain bacteria and fungi. Second, this ammonium is then converted to nitrites (NO2−) by nitrifying bacteria, such as Nitrosomonas, through nitrification. Subsequently, nitrites are converted to nitrates (NO3−) by similar organisms. Lastly, the process of denitrification occurs, whereby bacteria, such as Pseudomonas and Clostridium, convert the nitrates into nitrogen gas, thus allowing it to re-enter the atmosphere.
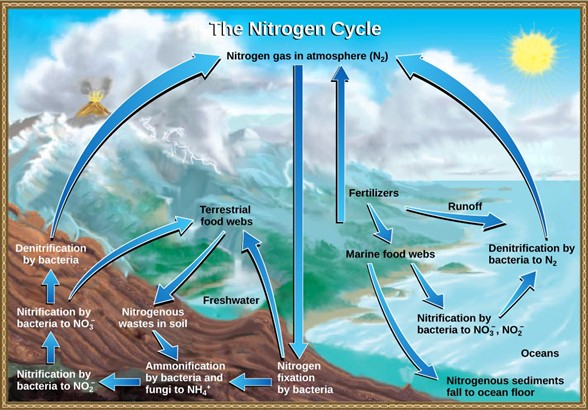
Figure 11: Nitrogen enters the living world from the atmosphere through nitrogen-fixing bacteria. This nitrogen and nitrogenous waste from animals is then processed back into gaseous nitrogen by soil bacteria, which also supply terrestrial food webs with the organic nitrogen they need. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases different nitrogen oxides, and by the use of artificial fertilizers (which contain nitrogen and phosphorus compounds) in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen (other than N2) is associated with several effects on Earth’s ecosystems including the production of acid rain (as nitric acid, HNO3) and greenhouse gas effects (as nitrous oxide, N2O), potentially causing climate change. A major effect from fertilizer runoff is saltwater and freshwater eutrophication, a process whereby nutrient runoff causes the overgrowth of algae and a number of consequential problems.
A similar process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria and archaea. Some of this nitrogen falls to the ocean floor as sediment, which can then be moved to land in geologic time by uplift of Earth’s surface, and thereby incorporated into terrestrial rock. Although the movement of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, there are data showing that this process may indeed be significant and should be included in any study of the global nitrogen cycle (Scott et al 2011).
Watch this video which deals with nitrogen pollution. You will see a connection to eutrophication, but there is more to it than that.
The Phosphorus Cycle
Phosphorus is an essential nutrient for living processes; it is a major component of nucleic acids and phospholipids, and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic, particularly freshwater, ecosystems.
Phosphorus occurs in nature as the phosphate ion (PO43–). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of the Earth’s surface (Fig 12).
Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine organisms. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
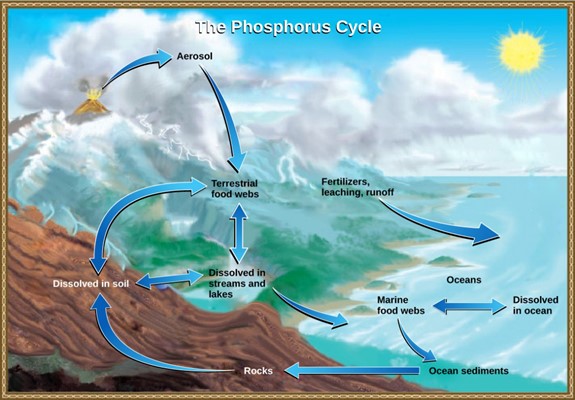
Figure 12: In nature, phosphorus exists as the phosphate ion (PO43-). Weathering of rocks and volcanic activity releases phosphate into the soil, water, and air, where it becomes available to terrestrial food webs. Phosphate enters the oceans in surface runoff, groundwater flow, and river flow. Phosphate dissolved in ocean water cycles into marine food webs. Some phosphate from the marine food webs falls to the ocean floor, where it forms sediment. (credit: modification of work by John M. Evans and Howard Perlman, USGS)
Excess phosphorus and nitrogen that enter these ecosystems from fertilizer runoff and from sewage cause excessive growth of algae. The subsequent death and decay of these organisms depletes dissolved oxygen, which leads to the death of aquatic organisms, such as shellfish and finfish. This process is responsible for dead zones in lakes and at the mouths of many major rivers and for massive fish kills, which often occur during the summer months (Fig 13).
A dead zone is an area in lakes and oceans near the mouths of rivers where large areas are periodically depleted of their normal flora and fauna; these zones can be caused by eutrophication, oil spills, dumping toxic chemicals, and other human activities. The number of dead zones has increased for several years, and more than 400 of these zones were present as of 2008. One of the worst dead zones is off the coast of the United States in the Gulf of Mexico: fertilizer runoff from the Mississippi River basin created a dead zone of over 8,463 square miles. Phosphate and nitrate runoff from fertilizers also negatively affect several lake and bay ecosystems including the Chesapeake Bay in the eastern United States.
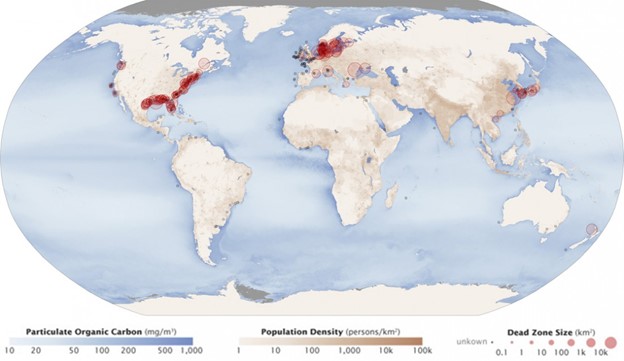
Figure 13: Dead zones occur when phosphorus and nitrogen from fertilizers cause excessive growth of microorganisms, which depletes oxygen and kills fauna. Worldwide, large dead zones are found in areas of high population density. (credit: Robert Simmon, Jesse Allen, NASA Earth Observatory)
This is a nice TED-Ed video on dead zones, nutrients, and provides a nice review of eutrophication as well.
Summary
Biogeochemical cycles, or nutrient cycles, are critical processes that describe the movement and transformation of essential elements and compounds through Earth’s ecosystems. These cycles play a fundamental role in maintaining the balance of nutrients and sustaining life on our planet and will cycle from abiotic components of the environment, such as the ocean or atmosphere, to biotic components, such as found in the proteins of plants. The carbon cycle involves the movement of carbon primarily between the ocean (a source or pool) to plants (a sink). The atmosphere also contains carbon and is critical for regulating Earth’s climate as well as a source for plants to pull out carbon and incorporate into organic compounds, such as sugars during photosynthesis. The nitrogen cycle focuses on the transformation of nitrogen gas (N2) into forms that can be used by plants, ultimately entering the food chain, as proteins and nucleic acids. And the phosphorus cycle, a much slower cycle than the others, centers around the movement of phosphorus through soil, water, and living organisms, especially important in plant growth. It is so slow because it primarily relies on the erosion of rocks to release phosphorus.
Human activities, such as burning fossil fuels, deforestation, and industrial agriculture (e.g. adding fertilizers to land), can disrupt biogeochemical cycles. For example, the burning of fossil fuels releases excess carbon dioxide into the atmosphere, contributing to global climate change. Pollution from agriculture can lead to nutrient imbalances in water bodies, causing problems like eutrophication.
These nutrients once embedded in living organisms, like plants, can make their way into other living organisms, like heterotrophs by moving through the food chain. Energy also moves through ecosystems but in only one direction. On average, about 10% of energy will move from one trophic level to the next. Most of the energy within a trophic level is used for maintained by those organisms, lost as heat during conversions or cannot be assimilated by the higher trophic level organisms.
If a pollutant, such as PCB, is moved along a food chain, it can accumulate in the fat tissue of organisms. Because pollutants tend to be stable and do not break down easily in the environment or in organisms, they can persist in living tissue. This process called bioaccumulation can lead to high levels of pollutants found in animals of higher trophic levels. This can be dangerous to those organisms or to other animals who might eat those animals.
Questions
Glossary
References
Jones, C. 2023. Biology and the Citizen. Utah State University
Kosal, E. 2023. Eutrophication section and Summary. NC State University
Scott L. Morford, Benjamin Z. Houlton, and RA Dahlgren. 2011. Increased Forest Ecosystem Carbon and Nitrogen Storage from Nitrogen Rich Bedrock. Nature 477 (7362): 78–81.

