6.11 A Comparison Between Biological Reactions and Laboratory Reactions
Beginning in the next chapter, we’ll be seeing a lot of reactions, some that are important in laboratory chemistry yet don’t occur in nature and others that have counterparts in biological pathways. In comparing laboratory reactions with biological reactions, several differences are apparent. For one, laboratory reactions are usually carried out in an organic solvent such as diethyl ether or dichloromethane to dissolve the reactants and bring them into contact, whereas biological reactions occur in the aqueous medium within cells. For another, laboratory reactions often take place over a wide range of temperatures without catalysts, while biological reactions take place at the temperature of the organism and are catalyzed by enzymes.
We’ll look at enzymes in more detail in Section 26.10, but you may already be aware that an enzyme is a large, globular, protein molecule that contains in its structure a protected pocket called its active site. The active site is lined by acidic or basic groups as needed for catalysis and has precisely the right shape to bind and hold a substrate molecule in the orientation necessary for reaction. Figure 6.10 shows a molecular model of hexokinase, along with an X-ray crystal structure of the glucose substrate and adenosine diphosphate (ADP) bound in the active site. Hexokinase is an enzyme that catalyzes the initial step of glucose metabolism—the transfer of a phosphate group from ATP to glucose, giving glucose 6-phosphate and ADP. The structures of ATP and ADP were shown at the end of Section 6.8.
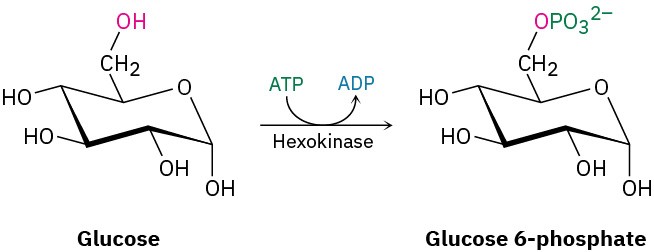
Note how the hexokinase-catalyzed phosphorylation reaction of glucose is written. It’s common when writing biological equations to show only the structures of the primary reactant and product, while abbreviating the structures of various biological “reagents” and by-products such as ATP and ADP. A curved arrow intersecting the straight reaction arrow indicates that ATP is also a reactant and ADP also a product.
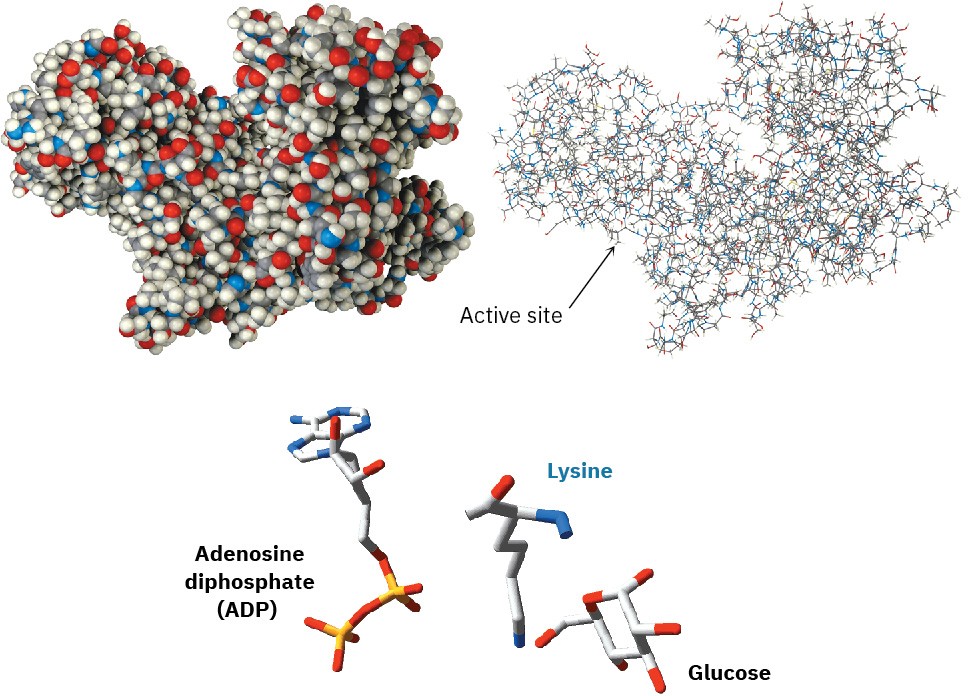
Figure 6.10 Models of hexokinase in space-filling and wire-frame formats, showing the cleft that contains the active site where substrate binding and reaction catalysis occur. At the bottom is an X-ray crystal structure of the enzyme active site, showing the positions of both glucose and ADP as well as a lysine amino acid that acts as a base to deprotonate glucose.
Yet a third difference between laboratory and biological reactions is that laboratory reactions are often done using relatively small, simple reagents such as Br2, HCl, NaBH4, CrO3, and so forth, while biological reactions usually involve relatively complex “reagents” called coenzymes. In the hexokinase-catalyzed phosphorylation of glucose just shown, ATP is the coenzyme. As another example, compare the H2 molecule, a laboratory reagent that adds to a carbon–carbon double bond to yield an alkane, with the reduced nicotinamide adenine dinucleotide (NADH) molecule, a coenzyme that effects an analogous addition of hydrogen to a double bond in many biological pathways. Of all the atoms in the coenzyme, only the one hydrogen atom shown in red is transferred to the double-bond substrate.
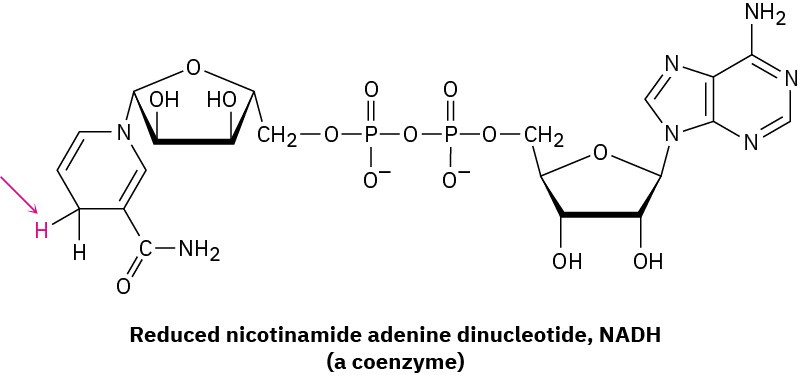
Don’t be intimidated by the size of the ATP or NADH molecule; most of the structure is there to provide an overall shape for binding to the enzyme and to provide appropriate solubility behavior. When looking at biological molecules, focus on the small part of the molecule where the chemical change takes place.
One final difference between laboratory and biological reactions is in their specificity. A catalyst might be used in the laboratory to catalyze the reaction of thousands of different substances, but an enzyme, because it can only bind a specific substrate molecule having a specific shape, will usually catalyze only a specific reaction. It’s this exquisite specificity that makes biological chemistry so remarkable and that makes life possible. Table 6.4 summarizes some of the differences between laboratory and biological reactions.
Table 6.4 A Comparison of Typical Laboratory and Biological Reactions A Comparison of Typical Laboratory and Biological Reactions
|
|
Biological reaction |
|
|
Solvent |
Organic liquid, such as ether |
Aqueous environment in cells |
|
Temperature |
Wide range; −80 to 150 °C |
Temperature of organism |
|
Catalyst |
Either none, or very simple |
Large, complex enzymes needed |
|
Reagent size |
Usually small and simple |
Relatively complex coenzymes |
|
Specificity |
Little specificity for substrate |
Very high specificity for substrate |
Additional Problems 6 • Additional Problems 6 • Additional Problems Visualizing Chemistry Problem 6-14
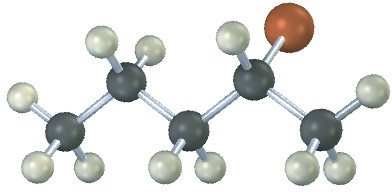
The following alkyl halide can be prepared by addition of HBr to two different alkenes. Draw the structures of both (reddish-brown = Br).
Problem 6-15
The following structure represents the carbocation intermediate formed in the addition reaction of HBr to two different alkenes. Draw the structures of both.
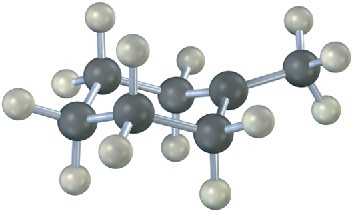
Problem 6-16
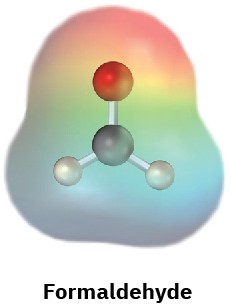
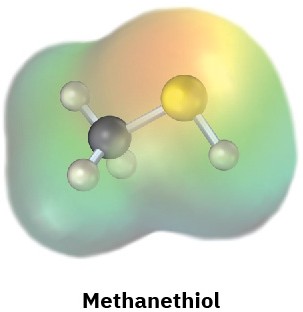
Electrostatic potential maps of (a) formaldehyde (CH2O) and (b) methanethiol (CH3SH) are shown. Is the formaldehyde carbon atom likely to be electrophilic or nucleophilic? What about the methanethiol sulfur atom? Explain.
(a)
(b)
Problem 6-17
Look at the following energy diagram:
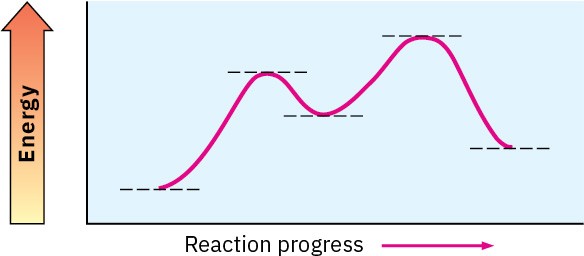
(a)
Is ΔG° for the reaction positive or negative? Label it on the diagram. (b)
How many steps are involved in the reaction? (c)
How many transition states are there? Label them on the diagram. Problem 6-18
Look at the following energy diagram for an enzyme-catalyzed reaction:
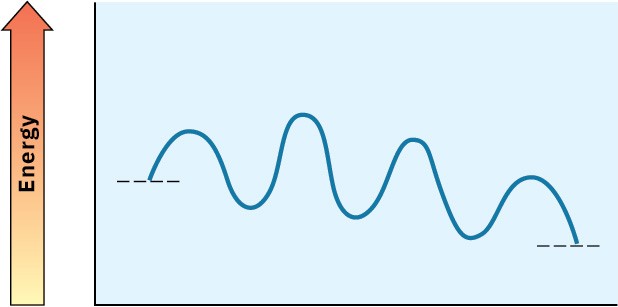
(a)
How many steps are involved?
(b)
Which step is most exergonic? (c)
Which step is slowest?
Energy Diagrams and Reaction Mechanisms
Problem 6-19
What is the difference between a transition state and an intermediate? Problem 6-20
Draw an energy diagram for a one-step reaction with Keq < 1. Label the parts of the diagram corresponding to reactants, products, transition state, ΔG°, and ΔG‡. Is ΔG° positive or negative?
Problem 6-21
Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate. Is ΔG° positive or negative?
Problem 6-22
Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step.
Problem 6-23
Draw an energy diagram for a reaction with Keq = 1. What is the value of ΔG° in this reaction?
Problem 6-24
The addition of water to ethylene to yield ethanol has the following thermodynamic parameters:
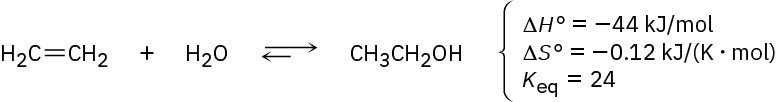
(a)
Is the reaction exothermic or endothermic? (b)
Is the reaction favorable (spontaneous) or unfavorable (nonspontaneous) at room temperature (298 K)?
Problem 6-25
When isopropylidenecyclohexane is treated with strong acid at room temperature, isomerization occurs by the mechanism shown below to yield 1-isopropylcyclohexene:
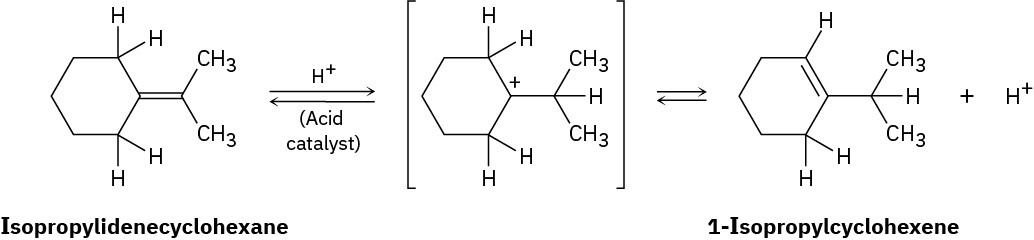
At equilibrium, the product mixture contains about 30% isopropylidenecyclohexane and about 70% 1-isopropylcyclohexene.
(a)
What is an approximate value of Keq for the reaction? (b)
Since the reaction occurs slowly at room temperature, what is its approximate ΔG‡? (c)
Draw an energy diagram for the reaction. Problem 6-26
Add curved arrows to the mechanism shown in Problem 6-25 to indicate the electron movement in each step.
Problem 6-27
Draw the complete mechanism for each of the following polar reactions. (a)
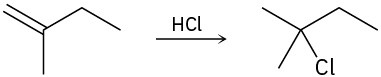
(b)
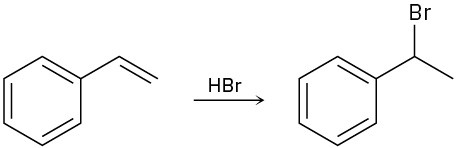
(c)
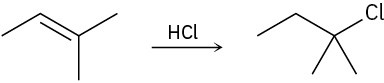
Problem 6-28
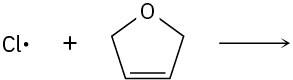
Use curved arrows to show the flow of electrons, and draw the carbon radical that is formed when the halogen radicals below add to the corresponding alkenes.
(a)

(b)
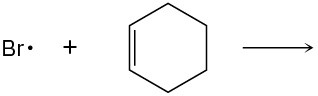
(c)
Polar Reactions
Problem 6-29
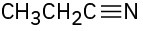 Identify the functional groups in the following molecules, and show the polarity of each: (a)
Identify the functional groups in the following molecules, and show the polarity of each: (a)
(b)
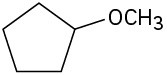
(c)
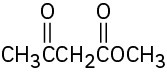
(d)
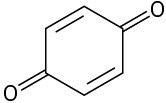
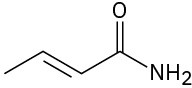
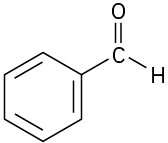
Problem 6-30
Identify the following reactions as additions, eliminations, substitutions, or rearrangements:
(a)
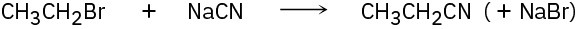
(b)
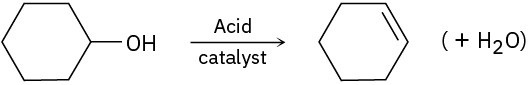
(c)
(d)
Problem 6-31
 Identify the likely electrophilic and nucleophilic sites in each of the following molecules: (a)
Identify the likely electrophilic and nucleophilic sites in each of the following molecules: (a)
(b)
Problem 6-32
Identify the electrophile and the nucleophile. (a)

(b)
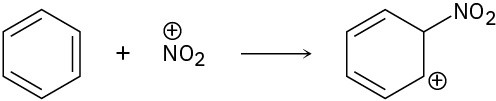
(c)
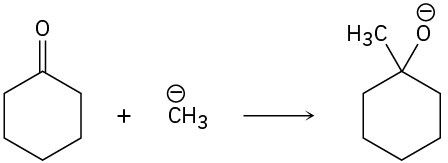
Problem 6-33
Add curved arrows to the following polar reactions to indicate the flow of electrons in each: (a)
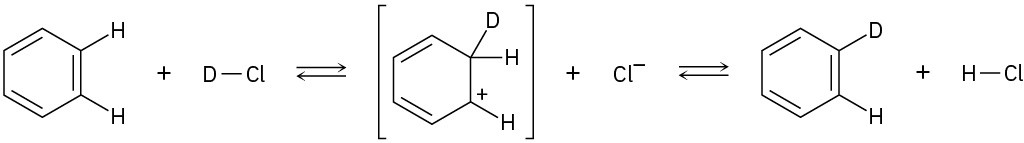
(b)
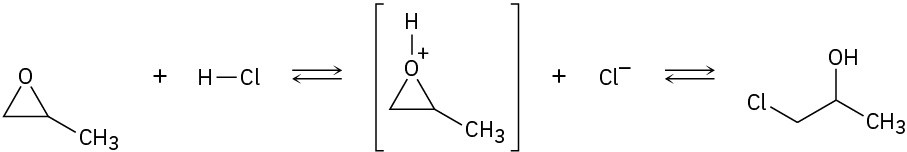
Problem 6-34
Follow the flow of electrons indicated by the curved arrows in each of the following polar reactions, and predict the products that result:
(a)
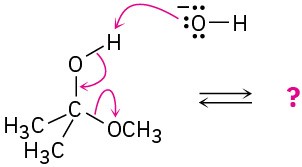
(b)
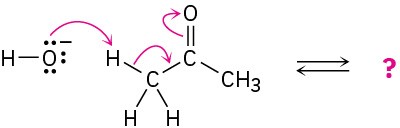
(c)
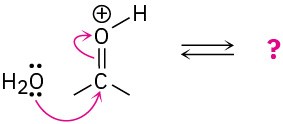
(d)
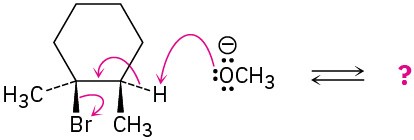
Radical Reactions
Problem 6-35
Radical chlorination of pentane is a poor way to prepare 1-chloropentane, but radical chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride, (CH3)3CCH2Cl. Explain.
Problem 6-36
Despite the limitations of radical chlorination of alkanes, the reaction is still useful for synthesizing certain halogenated compounds. For which of the following compounds does radical chlorination give a single monochloro product?
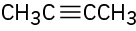 (a)(b)(c)
(a)(b)(c) 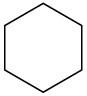 (d)
(d) 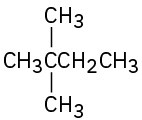 (e)
(e)
(f)
Problem 6-37
Draw the different monochlorinated constitutional isomers you would obtain by the radical chlorination of the following compounds.
(a)
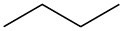
(b)
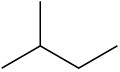
(c)
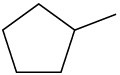
Problem 6-38
Answer question 6-37 taking all stereoisomers into account. Problem 6-39
Show the structure of the carbocation that would result when each of the following alkenes reacts with an acid, H+.
(a)
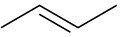
(b)
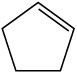
(c)
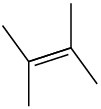
General Problems
Problem 6-40
2-Chloro-2-methylpropane reacts with water in three steps to yield 2-methyl-2-propanol. The first step is slower than the second, which in turn is much slower than the third. The reaction takes place slowly at room temperature, and the equilibrium constant is approximately 1.
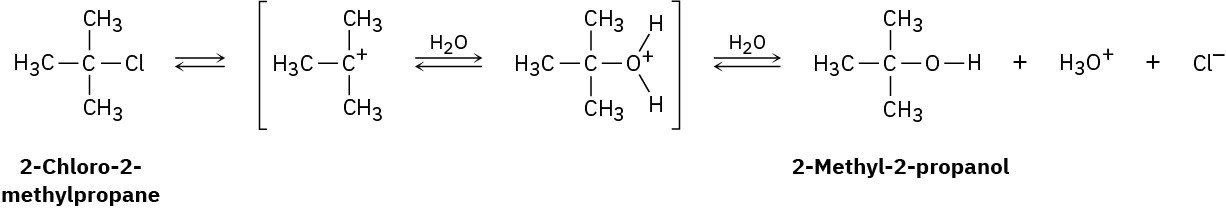
(a)
Give approximate values for ΔG‡ and ΔG° that are consistent with the above information. (b)
Draw an energy diagram for the reaction, labeling all points of interest and placing relative energy levels on the diagram consistent with the information given.
Problem 6-41
Add curved arrows to the mechanism shown in Problem 6-40 to indicate the electron movement in each step.
Problem 6-42
The reaction of hydroxide ion with chloromethane to yield methanol and chloride ion is an example of a general reaction type called a nucleophilic substitution reaction:
HO− + CH3Cl ⇄ CH3OH + Cl−
The value of ΔH° for the reaction is −75 kJ/mol, and the value of ΔS° is +54 J/(K·mol). What is the value of ΔG° (in kJ/mol) at 298 K? Is the reaction exothermic or endothermic? Is it exergonic or endergonic?
Problem 6-43
Methoxide ion (CH3O−) reacts with bromoethane in a single step according to the following equation:
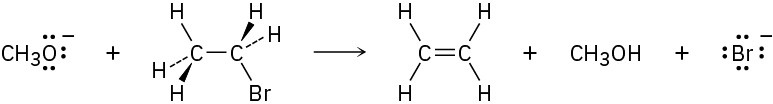
Identify the bonds broken and formed, and draw curved arrows to represent the flow of electrons during the reaction.
Problem 6-44
Ammonia reacts with acetyl chloride (CH3COCl) to give acetamide (CH3CONH2). Identify the bonds broken and formed in each step of the reaction, and draw curved arrows to represent the flow of electrons in each step.
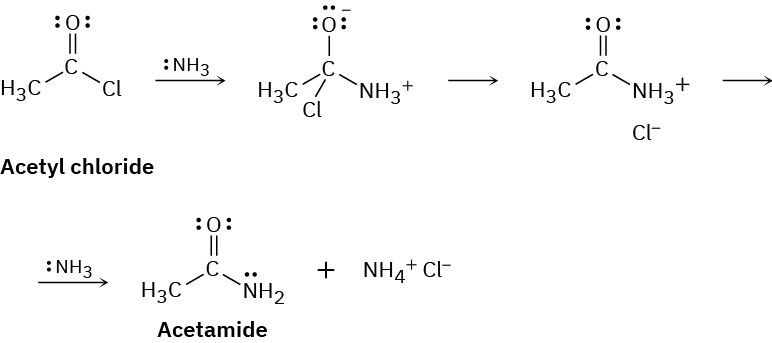
Problem 6-45
The naturally occurring molecule α-terpineol is biosynthesized by a route that includes the following step:
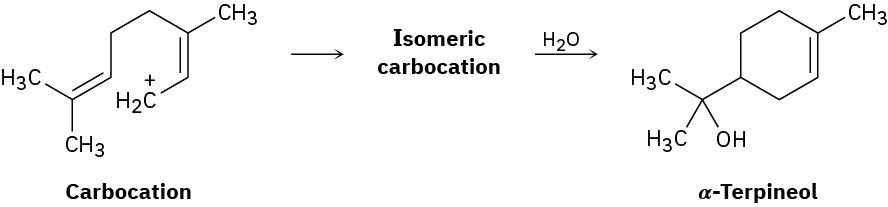
(a)
Propose a likely structure for the isomeric carbocation intermediate. (b)
Show the mechanism of each step in the biosynthetic pathway, using curved arrows to indicate electron flow.
Problem 6-46
Predict the product(s) of each of the following biological reactions by interpreting the flow of electrons as indicated by the curved arrows:
(a)
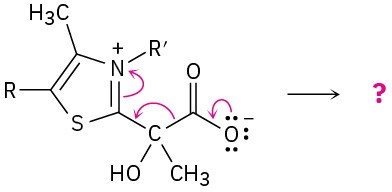
(b)
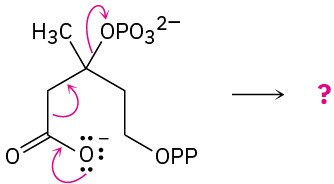
(c)
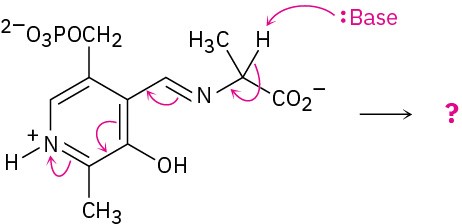
Problem 6-47
Reaction of 2-methylpropene with HBr might, in principle, lead to a mixture of two alkyl bromide addition products. Name them, and draw their structures.
Problem 6-48
Draw the structures of the two carbocation intermediates that might form during the reaction of 2-methylpropene with HBr (Problem 6-47). We’ll see in the next chapter that the stability of carbocations depends on the number of alkyl substituents attached to the positively charged carbon—the more alkyl substituents there are, the more stable the cation. Which of the two carbocation intermediates you drew is more stable?

