31.7 Polymer Structure and Physical Properties
Polymers aren’t really that different from other organic molecules. They’re much larger, of course, but their chemistry is similar to that of analogous small molecules. Thus, the alkane chains of polyethylene undergo radical-initiated halogenation, the aromatic rings of polystyrene undergo typical electrophilic aromatic substitution reactions, and the amide linkages of nylon are hydrolyzed by aqueous base.
The major difference between small and large organic molecules is in their physical properties. For instance, their large size means that polymers experience substantially greater van der Waals forces than do small molecules (Section 2.12). But because van der Waals forces operate only at close distances, they are strongest in polymers like high- density polyethylene, in which chains can pack together closely in a regular way. Many polymers, in fact, have regions that are essentially crystalline. These regions, called crystallites, consist of highly ordered portions in which the zigzag polymer chains are held together by van der Waals forces (Figure 31.4).
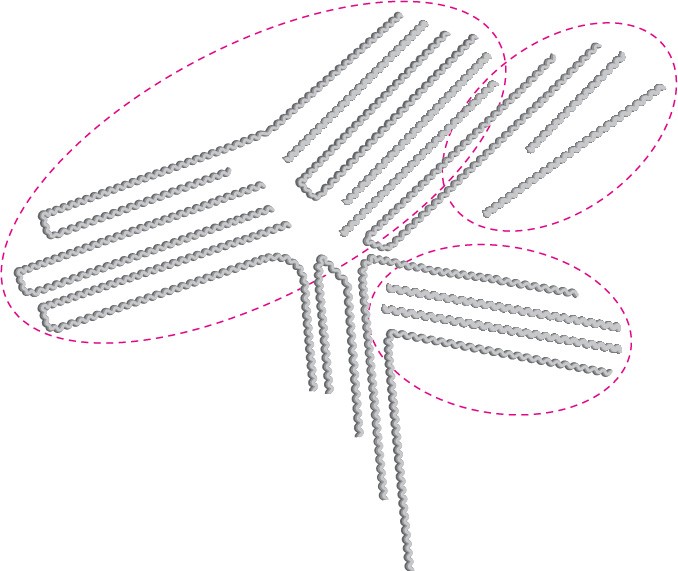
Figure 31.4 Crystallites in linear polyethylene. The long polymer chains are arranged in parallel lines in the crystallite regions.
As you might expect, polymer crystallinity is strongly affected by the steric requirements of substituent groups on the chains. Linear polyethylene is highly crystalline, but poly(methyl methacrylate) is noncrystalline because the chains can’t pack closely together in a regular way. Polymers with a high degree of crystallinity are generally hard and durable. When heated, the crystalline regions melt at the melt transition temperature, Tm, to give an amorphous material.
Noncrystalline, amorphous polymers like poly(methyl methacrylate), sold under the trade name Plexiglas, have little or no long-range ordering among chains but can nevertheless be
very hard at room temperature. When heated, the hard amorphous polymer becomes soft and flexible at a point called the glass transition temperature, Tg. Much of the art in polymer synthesis lies in finding methods for controlling the degree of crystallinity and the glass transition temperature, thereby imparting useful properties to the polymer.
In general, polymers can be divided into four major categories, depending on their physical behavior: thermoplastics, fibers, elastomers, and thermosetting resins. Thermoplastics are the polymers most people think of when the word plastic is mentioned. These polymers have a high glass transition temperature and are therefore hard at room temperature but become soft and viscous when heated. As a result, they can be molded into toys, beads, telephone housings, or any of a thousand other items. Because thermoplastics have little or no cross-linking, the individual chains can slip past one another in the melt. Some thermoplastic polymers, such as poly(methyl methacrylate) and polystyrene, are amorphous and noncrystalline; others, such as polyethylene and nylon, are partially crystalline. Among the better-known thermoplastics is poly(ethylene terephthalate), or PET, used for making plastic soft-drink bottles.
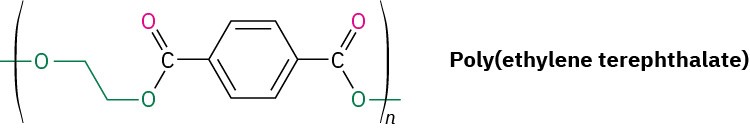
Plasticizers—small organic molecules that act as lubricants between chains—are usually added to thermoplastics to keep them from becoming brittle at room temperature. An example is poly(vinyl chloride), which is brittle when pure but becomes supple and pliable when a plasticizer is added. In fact, most drip bags used in hospitals to deliver intravenous saline solutions are made of poly(vinyl chloride), although replacements such as polypropylene are appearing.
Dialkyl phthalates such as di(2-ethylhexyl) phthalate (generally called dioctyl phthalate) are commonly used as plasticizers, although questions about their safety have been raised. The U.S. Food and Drug Administration (FDA) has advised the use of alternative materials in compromised patients and infants but has found no evidence of toxicity for healthy individuals. In addition, children’s toys that contain phthalates have been banned in the United States.
Fibers are thin threads produced by extruding a molten polymer through small holes in a die, or spinneret. The fibers are then cooled and drawn out, which orients the crystallite regions along the axis of the fiber and adds considerable tensile strength Figure 31.5.
Nylon, Dacron, and polyethylene all have the semicrystalline structure necessary for being drawn into oriented fibers.
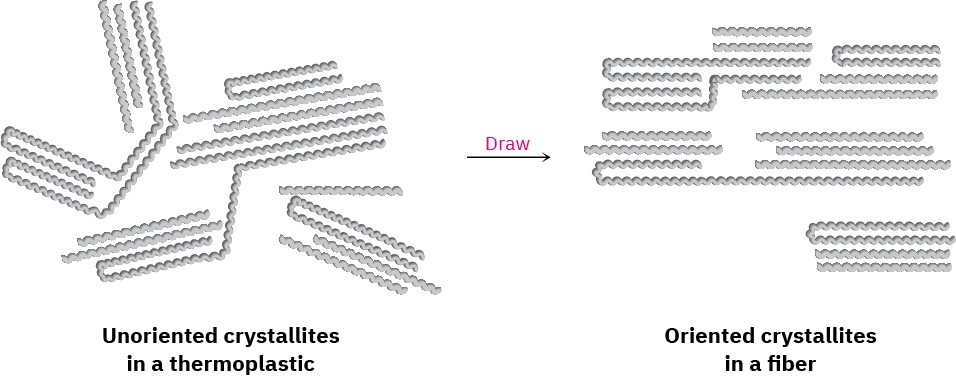
Figure 31.5Oriented crystallite regions in a polymer fiber.
Elastomers are amorphous polymers that have the ability to stretch out and spring back to their original shapes. These polymers must have low glass transition temperatures and a small amount of cross-linking to prevent the chains from slipping over one another. In addition, the chains must have an irregular shape to prevent crystallite formation. When stretched, the randomly coiled chains straighten out and orient along the direction of the pull. Van der Waals forces are too weak and too few to maintain this orientation, however, and the elastomer therefore reverts to its random coiled state when the stretching force is released (Figure 31.6).
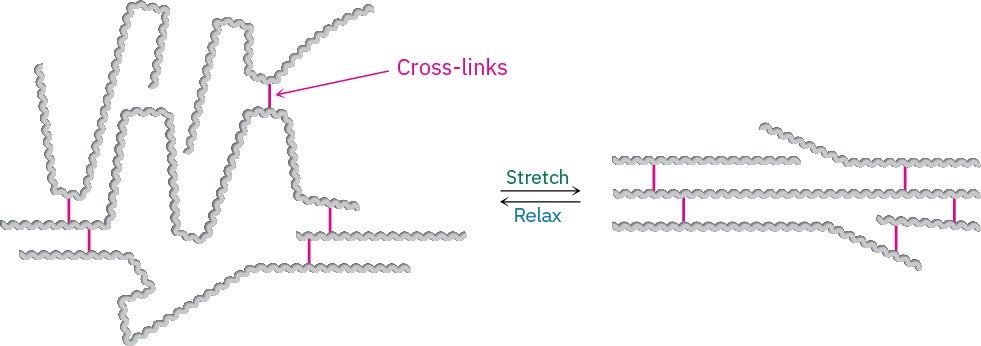
Figure 31.6Unstretched and stretched forms of an elastomer.
Natural rubber (Section 14.6) is the most common example of an elastomer. Rubber has the long chains and occasional cross-links needed for elasticity, but its irregular geometry prevents close packing of the chains into crystallites. Gutta-percha, by contrast, is highly crystalline and is not an elastomer (Figure 31.7).
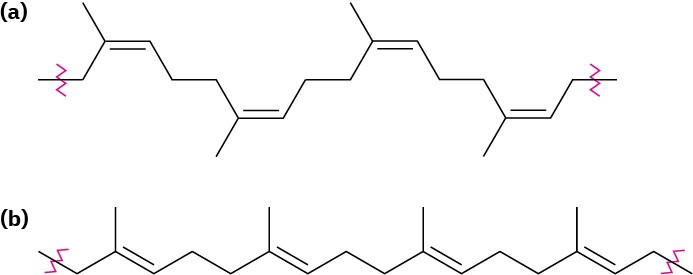
Figure 31.7(a) Natural rubber is elastic and noncrystalline because of its cis double-bond geometry, but (b) gutta-percha is nonelastic and crystalline because its geometry allows for better packing together of chains.
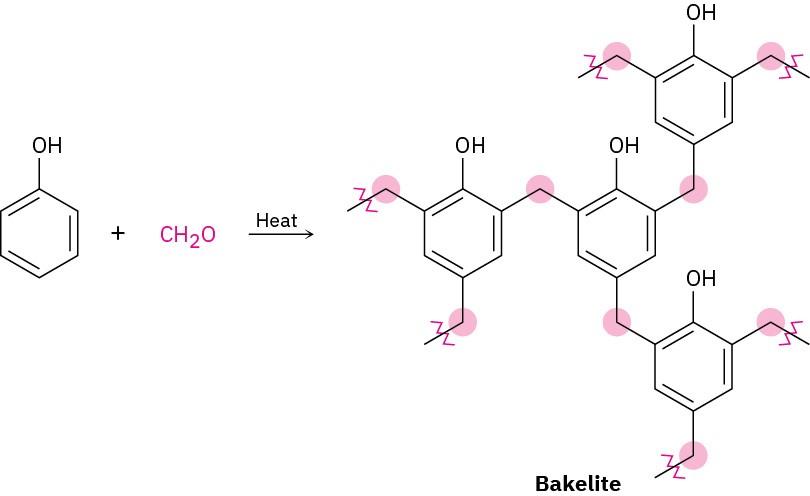
Thermosetting resins are polymers that become highly cross-linked and solidify into a hard, insoluble mass when heated. Bakelite, a thermosetting resin first produced in 1907, has been in commercial use longer than any other synthetic polymer. It is widely used for molded parts, adhesives, coatings, and even high-temperature applications such as missile nose cones.
Chemically, Bakelite is a phenolic resin, produced by reaction of phenol and formaldehyde. On heating, water is eliminated, many cross-links form, and the polymer sets into a rocklike mass. The cross-linking in Bakelite and other thermosetting resins is three-dimensional and is so extensive that we can’t really speak of polymer “chains.” A piece of Bakelite is essentially one large molecule.
Problem 31-11
What product would you expect to obtain from catalytic hydrogenation of natural rubber? Would the product be syndiotactic, atactic, or isotactic?
Problem 31-12
Propose a mechanism to account for the formation of Bakelite from acid-catalyzed polymerization of phenol and formaldehyde.
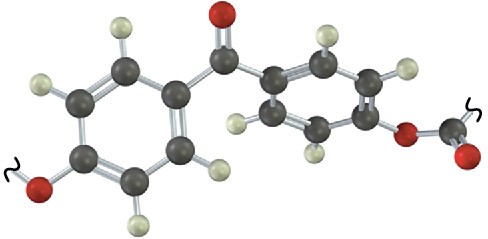
Additional Problems 31 • Additional Problems 31 • Additional Problems Visualizing Chemistry Problem 31-13
Identify the structural class to which the following polymer belongs, and show the structure of the monomer units used to make it:
Problem 31-14
Show the structures of the polymers that could be made from the following monomers (green = Cl):
(a)
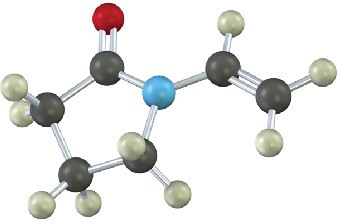
(b)
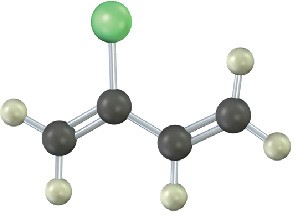
Mechanism Problems
Problem 31-15
Poly(ethylene glycol), or Carbowax, is made by anionic polymerization of ethylene oxide using NaOH as catalyst. Propose a mechanism.

Problem 31-16
The polyurethane foam used for home insulation uses methanediphenyldiisocyanate (MDI) as monomer. The MDI is prepared by acid-catalyzed reaction of aniline with formaldehyde, followed by treatment with phosgene, COCl2. Propose mechanisms for both steps.
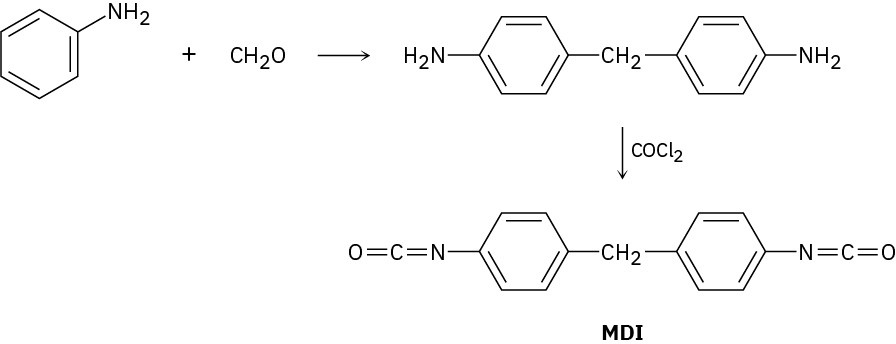
Problem 31-17
Write the structure of a representative segment of polyurethane prepared by reaction of ethylene glycol with MDI (Problem 31-16).
Problem 31-18
The polymeric resin used for Merrifield solid-phase peptide synthesis (Section 26.8) is prepared by treating polystyrene with N-(hydroxymethyl)phthalimide and trifluoromethanesulfonic acid, followed by reaction with hydrazine. Propose a mechanism for both steps.
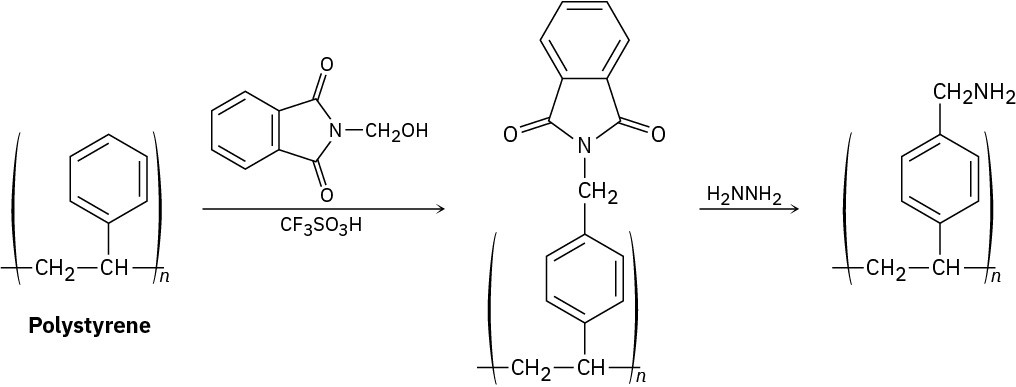
Problem 31-19
Polydicyclopentadiene (PDCPD), marketed as Telene or Metton, is a highly cross-linked thermosetting resin used for molding such impact-resistant parts as cabs for large trucks and earth-moving equipment. PDCPD is prepared by ring-opening metathesis polymerization of dicyclopentadiene, which is itself prepared from 1,3-cyclopentadiene. The polymerization occurs by initial metathesis of the more highly strained double bond in the bicyclo[2.2.1]heptane part of the molecule (Section 4.9) to give a linear polymer,
followed by cross-linking of different chains in a second metathesis of the remaining cyclopentene double bond.
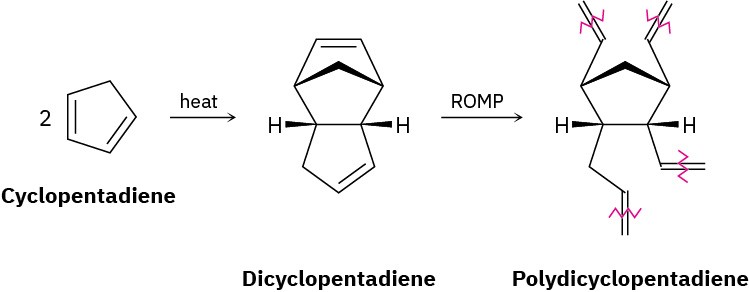
(a)
Show the mechanism of the formation of dicyclopentadiene from cyclopentadiene. (b)
Draw the structure of a representative sample of the initially formed linear polymer containing three monomer units.
(c)
Draw the structure of a representative sample of PDCPD that shows how cross-linking of the linear chains takes place.
General Problems
Problem 31-20
Identify the monomer units from which each of the following polymers is made, and tell whether each is a chain-growth or a step-growth polymer:
(a)
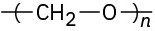
(b)
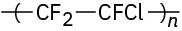
(c)
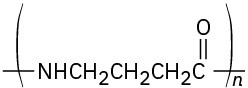
(d)
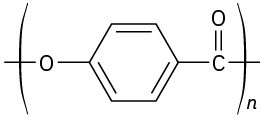
(e)
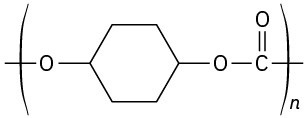
Problem 31-21
Draw a three-dimensional representation of segments of the following polymers: (a)
Syndiotactic polyacrylonitrile (b)
Atactic poly(methyl methacrylate) (c)
Isotactic poly(vinyl chloride) Problem 31-22
Draw the structure of Kodel, a polyester prepared by heating dimethyl 1,4- benzenedicarboxylate with 1,4-bis(hydroxymethyl)cyclohexane.
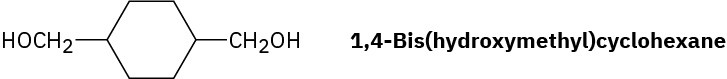
Problem 31-23
Show the structure of the polymer that results from heating the following diepoxide and diamine:
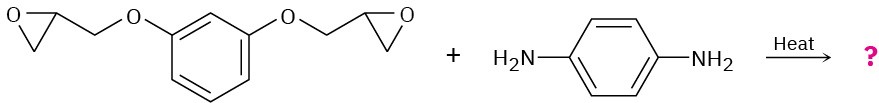
Problem 31-24
Nomex, a polyamide used in such applications as fire-retardant clothing, is prepared by reaction of 1,3-benzenediamine with 1,3-benzenedicarbonyl chloride. Show the structure of Nomex.
Problem 31-25
Nylon 10,10 is an extremely tough, strong polymer used to make reinforcing rods for concrete. Draw a segment of nylon 10,10, and show its monomer units.
Problem 31-26
1,3-Cyclopentadiene undergoes thermal polymerization to yield a polymer that has no double bonds in the chain. Upon strong heating, the polymer breaks down to regenerate cyclopentadiene. Propose a structure for the polymer.
Problem 31-27
When styrene, C6H5CH═CH2, is copolymerized in the presence of a few percent p– divinylbenzene, a hard, insoluble, cross-linked polymer is obtained. Show how this cross- linking of polystyrene chains occurs.
Problem 31-28
Nitroethylene, H2C═CHNO2, is a sensitive compound that must be prepared with great care. Attempted purification of nitroethylene by distillation often results in low recovery of product and a white coating on the inner walls of the distillation apparatus. Explain.
Problem 31-29
Poly(vinyl butyral) is used as the plastic laminate in the preparation of automobile windshield safety glass. How would you synthesize this polymer?
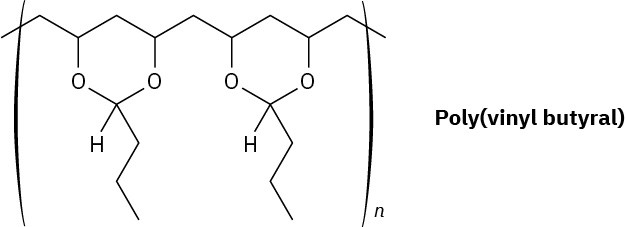
Problem 31-30
What is the structure of the polymer produced by anionic polymerization of β– propiolactone using NaOH as catalyst?
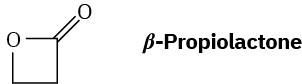
Problem 31-31
Glyptal is a highly cross-linked thermosetting resin produced by heating glycerol and phthalic anhydride (1,2-benzenedicarboxylic acid anhydride). Show the structure of a representative segment of glyptal.
Problem 31-32
Melmac, a thermosetting resin often used to make plastic dishes, is prepared by heating melamine with formaldehyde. Look at the structure of Bakelite shown in Section 31.7, and then propose a structure for Melmac.
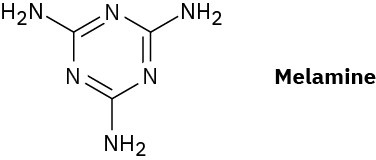
Problem 31-33
Epoxy adhesives are cross-linked resins prepared in two steps. The first step involves SN2 reaction of the disodium salt of bisphenol A with epichlorohydrin to form a low-molecular- weight prepolymer. This prepolymer is then “cured” into a cross-linked resin by treatment with a triamine such as H2NCH2CH2NHCH2CH2NH2.
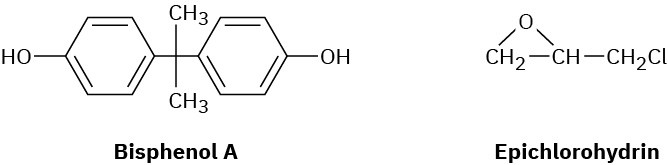
(a)
What is the structure of the prepolymer? (b)
How does addition of the triamine to the prepolymer result in cross-linking? Problem 31-34
The smoking salons of the Hindenburg and other hydrogen-filled dirigibles of the 1930s were insulated with urea–formaldehyde polymer foams. The structure of this polymer is highly cross-linked, like that of Bakelite (Section 31.7). Propose a structure.
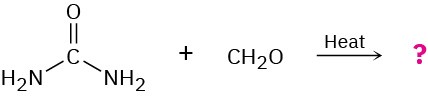
Problem 31-35
2-Ethyl-1-hexanol, used in the synthesis of di(2-ethylhexyl) phthalate plasticizer, is made commercially from butanal. Show the likely synthesis route.

