Chemistry Matters — Ethanol: Chemical, Drug, and Poison
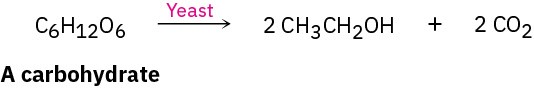
The production of ethanol by fermentation of grains and sugars is one of the oldest known organic reactions, going back at least 8000 years in the Middle East and perhaps as many as 9000 years in China. Fermentation is carried out by adding yeast to an aqueous sugar solution, where enzymes break down carbohydrates into ethanol and CO2. As noted in the chapter introduction, approximately 26 billion gallons of ethanol is produced each year by fermentation of corn and sugar, with essentially the entire amount used to make bus and automobile fuel.
Ethanol is classified medically as a central nervous system (CNS) depressant. Its effects— that is, being drunk—resemble the human response to anesthetics. There is an initial excitability and increase in sociable behavior, but this results from depression of inhibition rather than from stimulation. At a blood alcohol concentration of 0.1% to 0.3%, motor coordination is affected, accompanied by loss of balance, slurred speech, and amnesia.
When blood alcohol concentration rises to between 0.3% and 0.4%, nausea and loss of consciousness occur. Above 0.6%, spontaneous respiration and cardiovascular regulation are affected, ultimately leading to death. The LD50 of ethanol is 10.6 g/kg (Chapter 1 Chemistry Matters).
The passage of ethanol through the body begins with its absorption in the stomach and small intestine, followed by rapid distribution to all body fluids and organs. In the pituitary gland, ethanol inhibits the production of a hormone that regulates urine flow, causing increased urine production and dehydration. In the stomach, ethanol stimulates production of acid. Throughout the body, ethanol causes blood vessels to dilate, resulting in flushing of the skin and a sensation of warmth as blood moves into capillaries beneath the surface. The result is not a warming of the body, but an increased loss of heat at the surface.

Figure 17.16 The Harger Drunkometer was the first breath analyzer, introduced in 1938 to help convict drunk drivers. (credit: “Unidentified police officer with a Harger “Drunkometer” breathalyzer” by Florida Memory, State Library and Archives of Florida/Wikimedia Commons, Public Doman)
Ethanol metabolism occurs mainly in the liver and proceeds by oxidation in two steps, first to acetaldehyde (CH3CHO) and then to acetic acid (CH3CO2H). When continuously present in the body, ethanol and acetaldehyde are toxic, leading to the devastating physical and metabolic deterioration seen in people with chronic alcohol use disorder. The liver usually suffers the worst damage since it is the major site of alcohol metabolism.
Approximately 17,000 people are killed each year in the United States in alcohol-related automobile accidents. Thus, all 50 states have made it illegal to drive with a blood alcohol concentration (BAC) above 0.08%. Fortunately, simple tests have been devised for measuring blood alcohol concentration. The original breath analyzer test measured alcohol concentration in expired air by the color change occurring when the bright-orange oxidizing agent potassium dichromate (K2Cr2O7) reduced to blue-green chromium(III).
Current consumer devices use a conductivity sensor, and tests used by law-enforcement agencies use IR spectroscopy to measure blood-alcohol levels in expired air. Just breathe into the machine, and let the spectrum tell the tale.

