3.7 Conformations of Other Alkanes
Propane, the next-higher member in the alkane series, also has a torsional barrier that results in hindered rotation around the carbon–carbon bonds. The barrier is slightly higher in propane than in ethane—a total of 14 kJ/mol (3.4 kcal/mol) versus 12 kJ/mol.
The eclipsed conformation of propane has three interactions—two ethane-type hydrogen– hydrogen interactions and one additional hydrogen–methyl interaction. Since each eclipsing H ⟷ H interaction is the same as that in ethane and thus has an energy “cost” of
4.0 kJ/mol, we can assign a value of 14 – (2 × 4.0) = 6.0 kJ/mol (1.4 kcal/mol) to the eclipsing H ⟷ CH3 interaction (Figure 3.9).
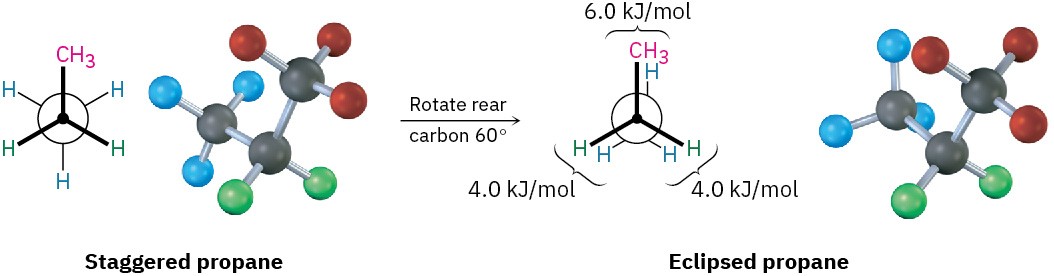
Figure 3.9 Newman projections of propane showing staggered and eclipsed conformations. The staggered conformer is lower in energy by 14 kJ/mol.
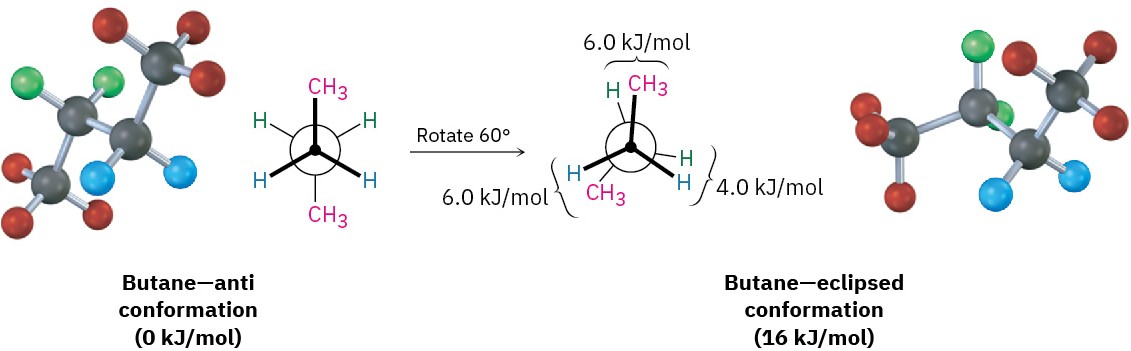
The conformational situation becomes more complex for larger alkanes because not all staggered conformations have the same energy and not all eclipsed conformations have the same energy. In butane, for instance, the lowest-energy arrangement, called the anti conformation, is the one in which the two methyl groups are as far apart as possible—180° away from each other. As rotation around the C2–C3 bond occurs, an eclipsed conformation is reached where there are two CH3 ⟷ H interactions and one H ⟷ H interaction. Using the energy values derived previously from ethane and propane, this eclipsed conformation is more strained than the anti conformation by 2 × 6.0 kJ/mol + 4.0 kJ/mol (two CH3 ⟷ H interactions plus one H ⟷ H interaction), for a total of 16 kJ/mol (3.8 kcal/mol).
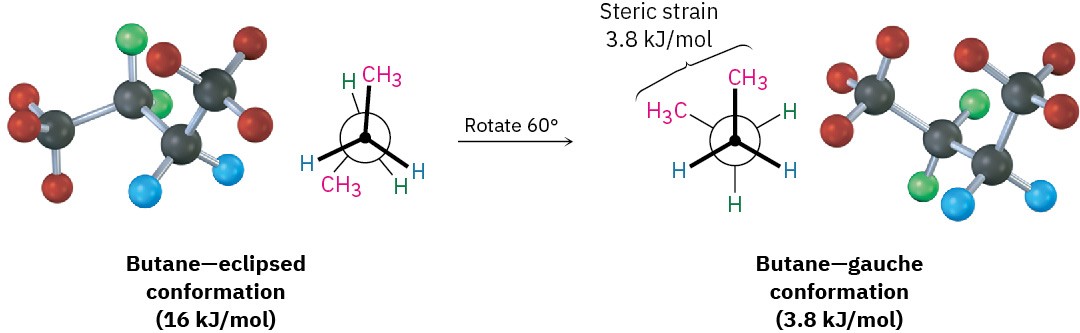
As bond rotation continues, an energy minimum is reached at the staggered conformation where the methyl groups are 60° apart. Called the gauche conformation, it lies 3.8 kJ/mol
(0.9 kcal/mol) higher in energy than the anti conformation even though it has no eclipsing interactions. This energy difference occurs because the hydrogen atoms of the methyl groups are near one another in the gauche conformation, resulting in what is called steric strain. Steric strain is the repulsive interaction that occurs when atoms are forced closer together than their atomic radii allow. It’s the result of trying to force two atoms to occupy the same space.
As the dihedral angle between the methyl groups approaches zero, an energy maximum is reached at a second eclipsed conformation. Because the methyl groups are forced even closer together than in the gauche conformation, both torsional strain and steric strain are present. A total strain energy of 19 kJ/mol (4.5 kcal/mol) has been estimated for this conformation, making it possible to calculate a value of 11 kJ/mol (2.6 kcal/mol) for the CH3 ⟷ CH3 eclipsing interaction: total strain of 19 kJ/mol minus the strain of two H ⟷ H eclipsing interactions (2 × 4.0 kcal/mol) equals 11 kJ/mol.
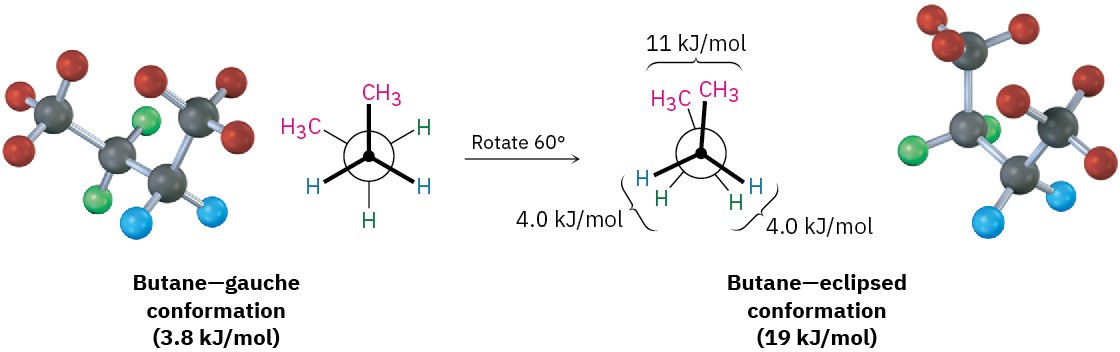
After 0°, the rotation becomes a mirror image of what we’ve already seen: another gauche conformation is reached, another eclipsed conformation, and finally a return to the anti conformation. A plot of potential energy versus rotation about the C2–C3 bond is shown in Figure 3.10.
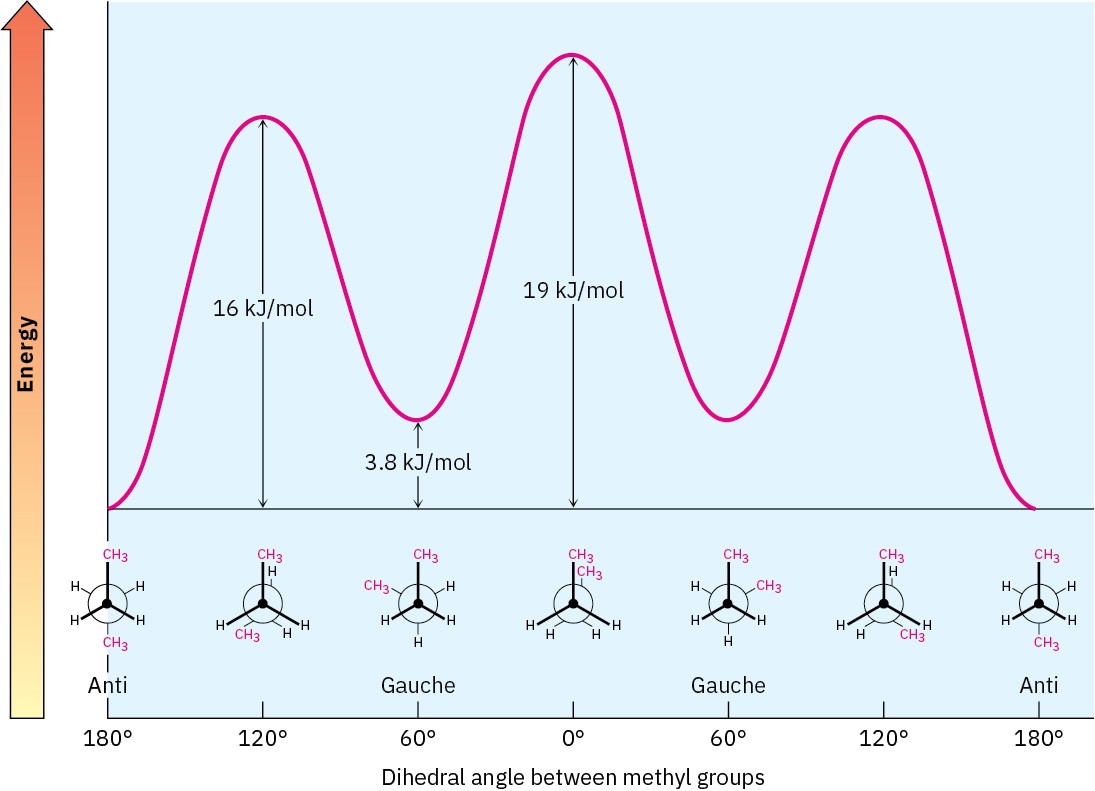
Figure 3.10 A plot of potential energy versus rotation for the C2−C3 bond in butane. The energy maximum occurs when the two methyl groups eclipse each other, and the energy minimum occurs when the two methyl groups are 180° apart (anti).
The notion of assigning definite energy values to specific interactions within a molecule is very useful, and we’ll return to it in the next chapter. A summary of what we’ve seen thus far is given in Table 3.5.
The same principles just developed for butane apply to pentane, hexane, and all higher alkanes. The most favorable conformation for any alkane has the carbon–carbon bonds in staggered arrangements, with large substituents arranged anti to one another. A generalized alkane structure is shown in Figure 3.11.
Table 3.5 Energy Costs for Interactions in Alkane Conformers
|
Cause |
Energy cost |
||
|
(kJ/mol) |
(kcal/mol) |
||
|
H ⟷ H eclipsed |
Torsional strain |
4.0 |
1.0 |
|
H ⟷ CH3 eclipsed |
Mostly torsional strain |
6.0 |
1.4 |
|
CH3 ⟷ CH3 eclipsed |
Torsional and steric strain |
11.0 |
2.6 |
|
CH3 ⟷ CH3 gauche |
Steric strain |
3.8 |
0.9 |
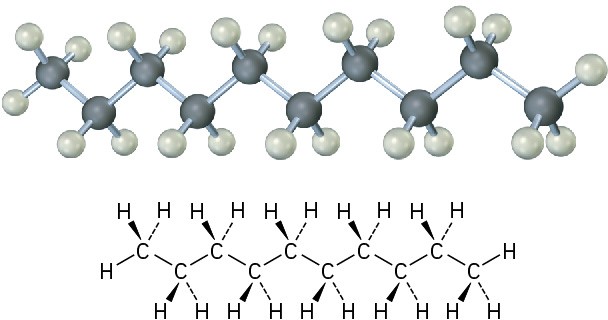
Figure 3.11 The most stable alkane conformation is the one in which all substituents are staggered and the carbon–carbon bonds are arranged anti, as shown in this model of decane.
One final point: saying that one particular conformer is “more stable” than another doesn’t mean the molecule adopts and maintains only the more stable conformation. At room temperature, rotations around σ bonds occur so rapidly that all conformers are in equilibrium. At any given instant, however, a larger percentage of molecules will be found in a more stable conformation than in a less stable one.
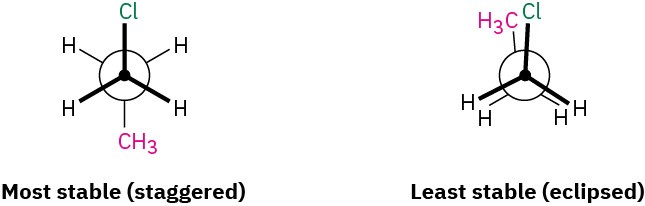
Worked Example 3.4Newman ProjectionsSight along the C1–C2 bond of 1-chloropropane, and draw Newman projections of the most stable and least stable conformations.StrategyThe most stable conformation of a substituted alkane is generally a staggered one in which large groups have an anti relationship. The least stable conformation is generally an eclipsed one in which large groups are as close as possible.Solution
Problem 3-15
Make a graph of potential energy versus angle of bond rotation for propane, and assign values to the energy maxima.
Problem 3-16
Sight along the C2–C1 bond of 2-methylpropane (isobutane). (a)
Draw a Newman projection of the most stable conformation. (b)
Draw a Newman projection of the least stable conformation. (c)
Make a graph of energy versus angle of rotation around the C2–C1 bond. (d)
Assign relative values to the maxima and minima in your graph, given that an H ⟷ H eclipsing interaction costs 4.0 kJ/mol and an H ⟷ CH3 eclipsing interaction costs 6.0 kJ/mol.
Problem 3-17
Sight along the C2–C3 bond of 2,3-dimethylbutane, and draw a Newman projection of the most stable conformation.
Problem 3-18
Draw a Newman projection along the C2–C3 bond of the following conformation of 2,3- dimethylbutane, and calculate a total strain energy:
Additional Problems 3 • Additional Problems 3 • Additional Problems Visualizing Chemistry Problem 3-19
Identify the functional groups in the following substances, and convert each drawing into a molecular formula (red = O, blue = N).
(a)
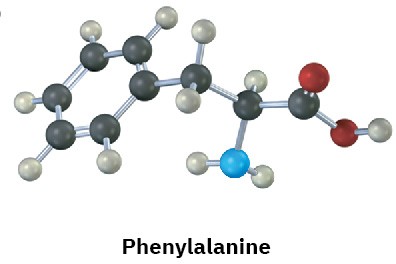
(b)
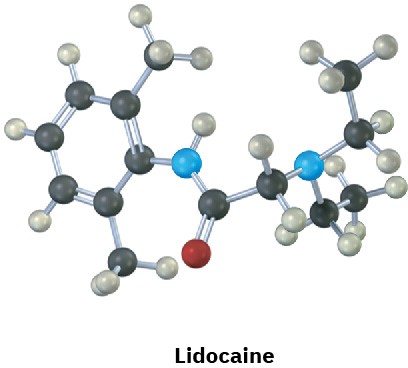
Problem 3-20
Give IUPAC names for the following alkanes, and convert each drawing into a skeletal structure.
(a)
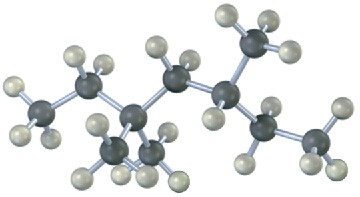
(b)
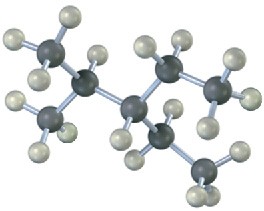
(c)
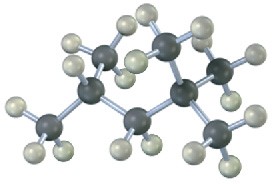
(d)
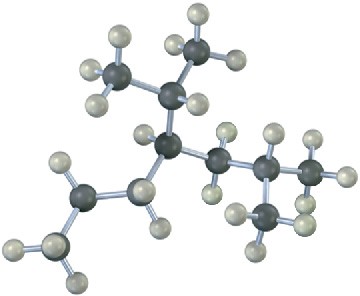
Problem 3-21
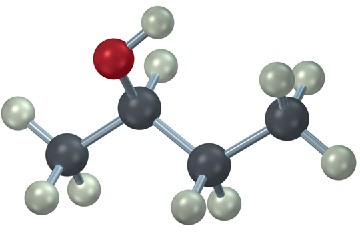
Draw a Newman projection along the C2–C3 bond of the following conformation of 2- butanol.
Functional Groups
Problem 3-22
Locate and identify the functional groups in the following molecules. (a)
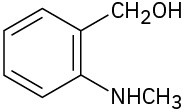
(b)
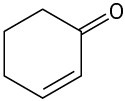
(c)
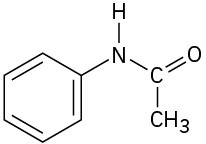
(d)
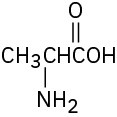
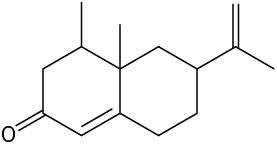
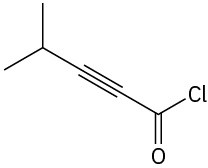
Problem 3-23
Propose structures that meet the following descriptions: (a)
A ketone with five carbons (b)
A four-carbon amide (c)
A five-carbon ester (d)
An aromatic aldehyde (e)
A keto ester (f)
An amino alcohol Problem 3-24
Propose structures for the following:
(a)
A ketone, C4H8O (b)
A nitrile, C5H9N (c)
A dialdehyde, C4H6O2 (d)
A bromoalkene, C6H11Br (e)
An alkane, C6H14 (f)
cyclic saturated hydrocarbon, C6H12 (g)
A diene (dialkene), C5H8 (h)
A keto alkene, C5H8O Problem 3-25
Predict the hybridization of the carbon atom in each of the following functional groups: (a)
Ketone (b) Nitrile (c)
Carboxylic acid Problem 3-26
Draw the structures of the following molecules:
(a)
Biacetyl, C4H6O2, a substance with the aroma of butter; it contains no rings or carbon– carbon multiple bonds.
(b)
Ethylenimine, C2H5N, a substance used in the synthesis of melamine polymers; it contains no multiple bonds.
(c)
Glycerol, C3H8O3, a substance isolated from fat and used in cosmetics; it has an –OH group on each carbon.
Isomers
Problem 3-27
Draw structures that meet the following descriptions (there are many possibilities): (a)
Three isomers with the formula C8H18 (b)
Two isomers with the formula C4H8O2 Problem 3-28
Draw structures of the nine isomers of C7H16. Problem 3-29
In each of the following sets, which structures represent the same compound and which represent different compounds?
(a)
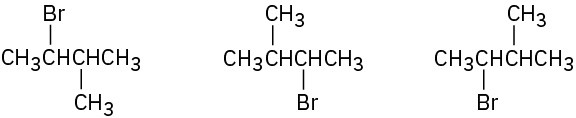
(b)
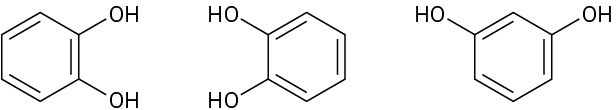
(c)
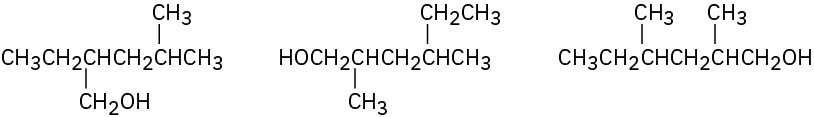
Problem 3-30
Seven constitutional isomers have the formula C4H10O. Draw as many as you can. Problem 3-31
Draw as many compounds as you can that fit the following descriptions: (a)
Alcohols with formula C4H10O (b)
Amines with formula C5H13N (c)
Ketones with formula C5H10O (d)
Aldehydes with formula C5H10O (e)
Esters with formula C4H8O2 (f)
Ethers with formula C4H10O Problem 3-32
Draw compounds that contain the following: (a)
A primary alcohol (b)
A tertiary nitrile (c)
A secondary thiol (d)
Both primary and secondary alcohols (e)
An isopropyl group (f)
A quaternary carbon Naming Compounds Problem 3-33
Draw and name all monobromo derivatives of pentane, C5H11Br. Problem 3-34
Draw and name all monochloro derivatives of 2,5-dimethylhexane, C8H17Cl. Problem 3-35
Draw structures for the following: (a)
2-Methylheptane (b)
4-Ethyl-2,2-dimethylhexane (c)
4-Ethyl-3,4-dimethyloctane (d)
2,4,4-Trimethylheptane (e)
3,3-Diethyl-2,5-dimethylnonane (f)
4-Isopropyl-3-methylheptane Problem 3-36
Draw a compound that:
(a)
Has only primary and tertiary carbons (b)
Has no secondary or tertiary carbons (c)
Has no secondary or tertiary carbons Problem 3-37
Draw a compound that:
(a)
Has nine primary hydrogens (b)
Has only primary hydrogens Problem 3-38
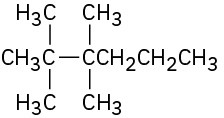
Give IUPAC names for the following compounds: (a)
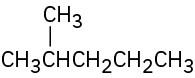
(b)
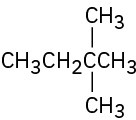
(c)
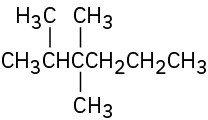
(d)
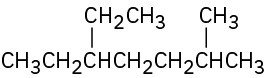
(e)
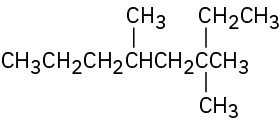
(f)
Problem 3-39
Name the five isomers of C6H14. Problem 3-40
Explain why each of the following names is incorrect: (a)
2,2-Dimethyl-6-ethylheptane (b)
- Ethyl-5,5-dimethylpentane (c)
3-Ethyl-4,4-dimethylhexane (d)
5,5,6-Trimethyloctane (e)
2-Isopropyl-4-methylheptane Problem 3-41
Propose structures and give IUPAC names for the following: (a)
A diethyldimethylhexane (b)
A (3-methylbutyl)-substituted alkane
Conformations
Problem 3-42
Consider 2-methylbutane (isopentane). Sighting along the C2–C3 bond: (a)
Draw a Newman projection of the most stable conformation. (b)
Draw a Newman projection of the least stable conformation. (c)
If a CH3 ⟷ CH3 eclipsing interaction costs 11 kJ/mol (2.5 kcal/mol) and a CH3 ⟷ CH3 gauche interaction costs 3.8 kJ/mol (0.9 kcal/mol), make a quantitative plot of energy versus rotation about the C2–C3 bond.
Problem 3-43
What are the relative energies of the three possible staggered conformations around the C2–C3 bond in 2,3-dimethylbutane? (See Problem 3-42.)
Problem 3-44
Construct a qualitative potential-energy diagram for rotation about the C–C bond of 1,2- dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2-dibromoethane.
Problem 3-45
Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is μ = 1.0 D. What does this tell you about the actual conformation of the molecule?
Problem 3-46
Draw the most stable conformation of pentane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.
Problem 3-47
Draw the most stable conformation of 1,4-dichlorobutane, using wedges and dashes to represent bonds coming out of the paper and going behind the paper, respectively.
General Problems
Problem 3-48
For each of the following compounds, draw an isomer that has the same functional groups. (a)
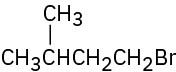
(b)
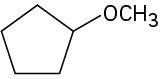
(c)
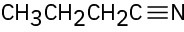
(d)
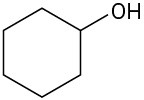
(e)
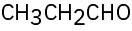
(f)
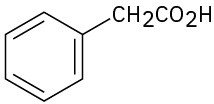
Problem 3-49
Malic acid, C4H6O5, has been isolated from apples. Because this compound reacts with 2 molar equivalents of base, it is a dicarboxylic acid.
(a)
Draw at least five possible structures. (b)
If malic acid is a secondary alcohol, what is its structure? Problem 3-50
Formaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative. When pure, formaldehyde trimerizes to give trioxane, C3H6O3, which, surprisingly enough, has no carbonyl groups. Only one monobromo derivative (C3H5BrO3) of trioxane is possible. Propose a structure for trioxane.
Problem 3-51
The barrier to rotation about the C–C bond in bromoethane is 15 kJ/mol (3.6 kcal/mol). (a)
What energy value can you assign to an H ↔ Br eclipsing interaction? (b)
Construct a quantitative diagram of potential energy versus bond rotation for bromoethane.
Problem 3-52
Increased substitution around a bond leads to increased strain. Take the four substituted butanes listed below, for example. For each compound, sight along the C2–C3 bond and draw Newman projections of the most stable and least stable conformations. Use the data in Table 3.5 to assign strain-energy values to each conformation. Which of the eight conformations is most strained? Which is least strained?
(a)
2-Methylbutane (b)
2,2-Dimethylbutane (c)
2,3-Dimethylbutane (d)
2,2,3-Trimethylbutane Problem 3-53
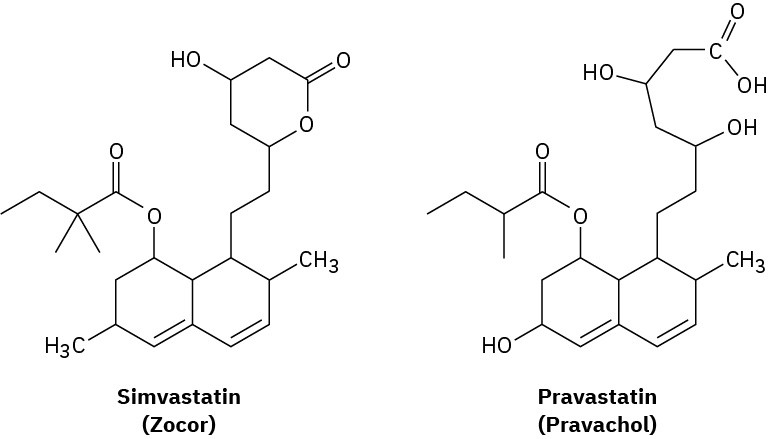
The cholesterol-lowering agents called statins, such as simvastatin (Zocor) and pravastatin (Pravachol), are among the most widely prescribed drugs in the world, with annual sales estimated at approximately $25 billion. Identify the functional groups in both, and tell how the two substances differ.
Problem 3-54
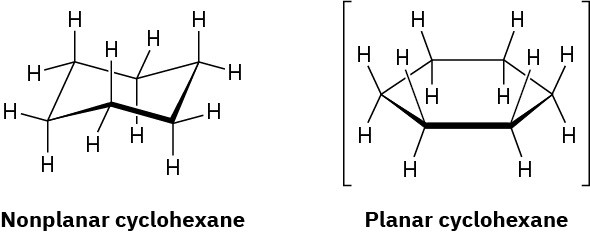
In the next chapter we’ll look at cycloalkanes—saturated cyclic hydrocarbons—and we’ll see that the molecules generally adopt puckered, nonplanar conformations. Cyclohexane, for instance, has a puckered shape like a lounge chair rather than a flat shape. Why?
Problem 3-55
We’ll see in the next chapter that there are two isomeric substances, both named 1,2- dimethylcyclohexane. Explain.


