20.8 Spectroscopy of Carboxylic Acids and Nitriles
Infrared Spectroscopy
Carboxylic acids have two characteristic IR absorptions that make the –CO2H group easily identifiable. The O–H bond of the carboxyl group gives rise to a very broad absorption over the range 2500 to 3300 cm–1. The C═O bond shows an absorption between 1710 and 1760 cm–1. The exact position of C═O absorption depends both on the structure of the molecule and on whether the acid is free (monomeric) or hydrogen-bonded (dimeric). Free carboxyl groups absorb at 1760 cm–1, but the more commonly encountered dimeric carboxyl groups absorb in a broad band centered around 1710 cm–1. As with other carbonyl-containing functional groups, conjugation with an alkene or benzene ring lowers the frequency of the C═O stretch by 20 to 30 cm–1.

Both the broad O–H absorption and the C═O absorption at 1710 cm–1 (dimeric) are identified in the IR spectrum of butanoic acid shown in Figure 20.6.
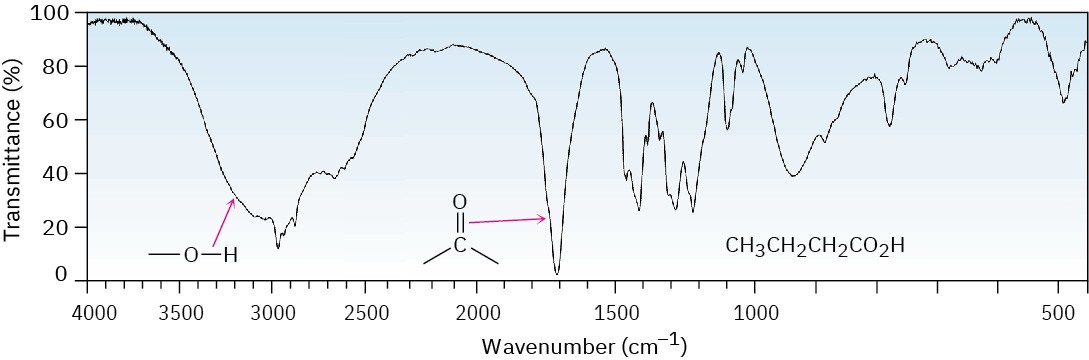
Figure 20.6IR spectrum of butanoic acid, CH3CH2CH2CO2H.
Nitriles show an intense and easily recognizable C≡N bond absorption near 2250 cm–1 for saturated compounds and 2230 cm–1 for aromatic and conjugated molecules. Few other functional groups absorb in this region, so IR spectroscopy is highly diagnostic for nitriles.
Problem 20-15
Cyclopentanecarboxylic acid and 4-hydroxycyclohexanone have the same formula (C6H10O2), and both contain an –OH and a C═O group. How could you distinguish between them using IR spectroscopy?
Nuclear Magnetic Resonance Spectroscopy
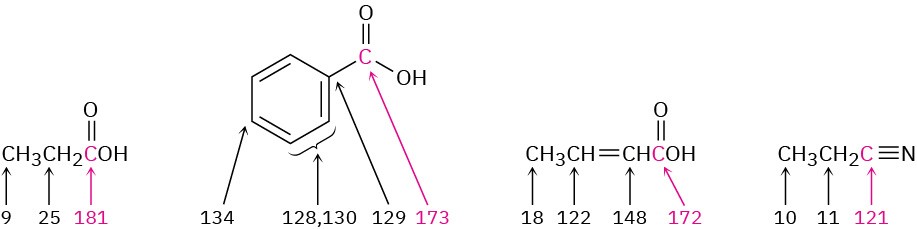
Carboxyl carbon atoms absorb in the range 165 to 185 δ in the 13C NMR spectrum, with aromatic and α,β-unsaturated acids near the upfield end of the range (∼165 δ) and saturated aliphatic acids near the downfield end (∼185 δ). Nitrile carbons absorb in the range 115 to 130 δ.
In the 1H NMR spectrum, the acidic –CO2H proton normally absorbs as a singlet near 12 δ. The chemical shift of the carboxyl proton is concentration and solvent dependent, as these variables can change the extent of hydrogen-bonding in the sample. In some cases, the carboxyl-proton resonance is broadened to the point of being nearly undetectable. Traces of water in the sample can exacerbate the situation. As with alcohols (Section 17.11), the – CO2H proton can be replaced by deuterium when D2O is added to the sample tube, causing the absorption to disappear from the NMR spectrum. Figure 20.7 shows the 1H NMR spectrum of phenylacetic acid. Note that the –CO2H absorption occurs at 12.0 δ.
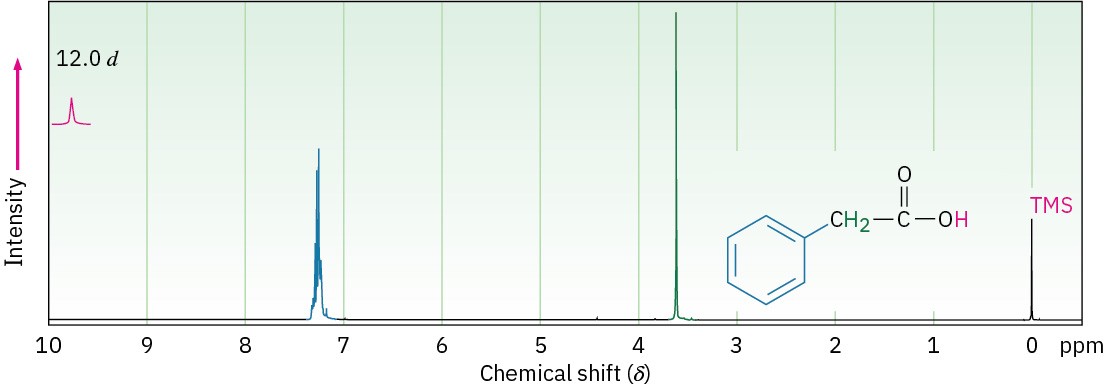
Figure 20.7Proton NMR spectrum of phenylacetic acid, C6H5CH2CO2H.
Problem 20-16
How could you distinguish between the isomers cyclopentanecarboxylic acid and 4- hydroxycyclohexanone by 1H and 13C NMR spectroscopy? (See Problem 20-15.)
Additional Problems 20 • Additional Problems 20 • Additional Problems Visualizing Chemistry Problem 20-17
Give IUPAC names for the following carboxylic acids (reddish brown = Br):
(a)
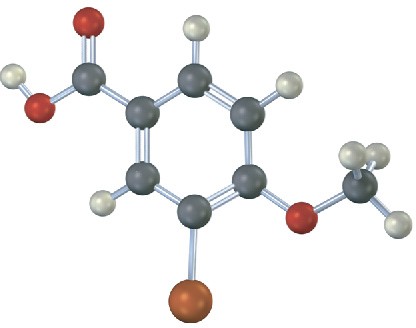
(b)
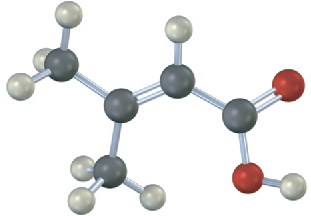
(c)
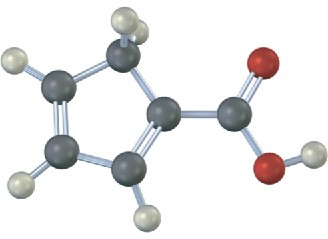
(d)
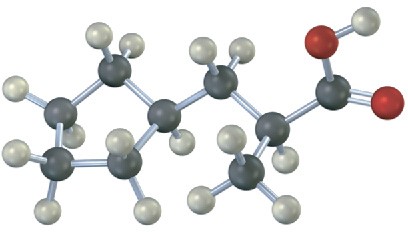
Problem 20-18
Would you expect the following carboxylic acids to be more acidic or less acidic than benzoic acid? Explain. (Reddish brown = Br.)
(a)
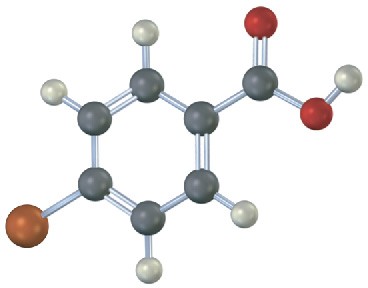
(b)
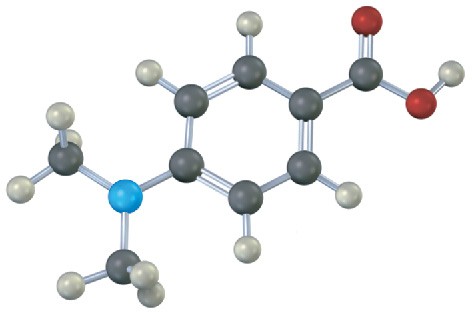
Problem 20-19
The following carboxylic acid can’t be prepared from an alkyl halide by either the nitrile hydrolysis route or the Grignard carboxylation route. Explain.
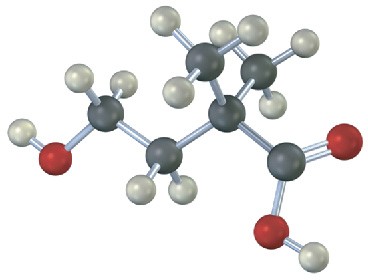
Problem 20-20
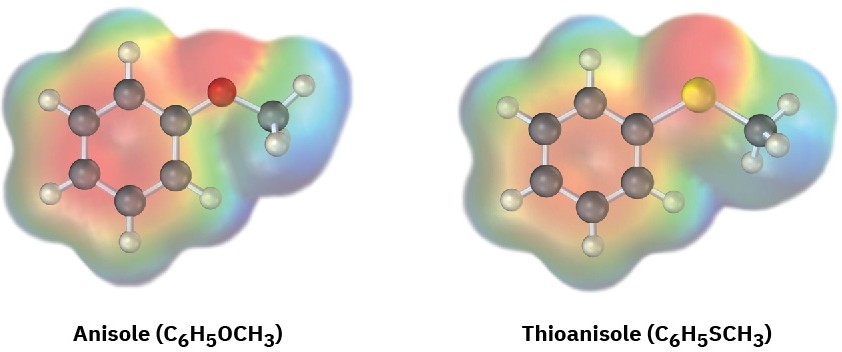
Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio)benzoic acid? Explain.
Mechanism Problems
Problem 20-21
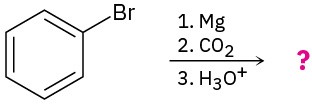 Predict the product(s) and write the mechanism of each of the following reactions: (a)
Predict the product(s) and write the mechanism of each of the following reactions: (a)
(b)
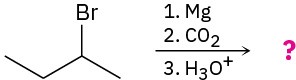
Problem 20-22
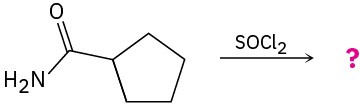 Predict the product(s) and write the mechanism of each of the following reactions: (a)
Predict the product(s) and write the mechanism of each of the following reactions: (a)
(b)
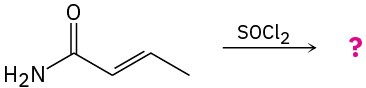
Problem 20-23
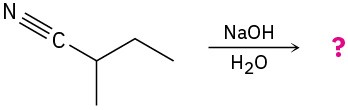 Predict the product(s) and write the mechanism of each of the following reactions: (a)
Predict the product(s) and write the mechanism of each of the following reactions: (a)
(b)
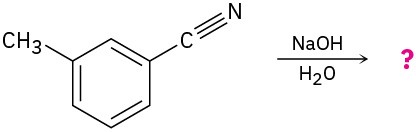
Problem 20-24
Predict the product(s) and write the complete mechanism of each of the following reactions:
(a)
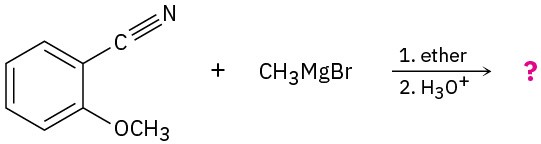
(b)
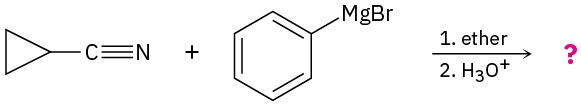
Problem 20-25
Acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of the nitrogen atom, followed by nucleophilic addition of water. Review the mechanism of base-catalyzed nitrile hydrolysis in Section 20.7 and then predict the products for the following reactions. Write the steps involved in the acid-catalyzed reaction, using curved arrows.
(a)
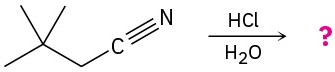
(b)
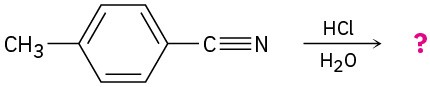
Problem 20-26
Nitriles can be converted directly to esters by the Pinner reaction, which first produces an iminoester salt that is isolated and then treated with water to give the final product.
Propose a mechanism for the Pinner reaction using curved arrows to show the flow of electrons at each step.
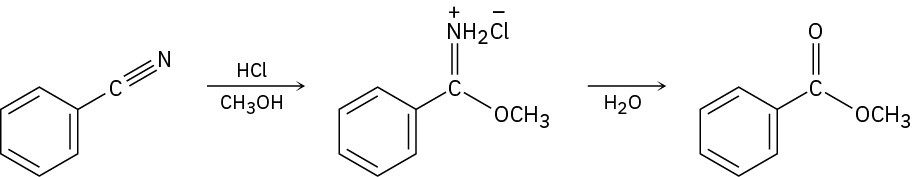
Problem 20-27
Naturally occurring compounds called cyanogenic glycosides, such as lotaustralin, release hydrogen cyanide, HCN, when treated with aqueous acid. The reaction occurs by hydrolysis of the acetal linkage to form a cyanohydrin, which then expels HCN and gives a carbonyl compound.
(a)
Show the mechanism of the acetal hydrolysis and the structure of the cyanohydrin that results.
(b)
Propose a mechanism for the loss of HCN, and show the structure of the carbonyl compound that forms.
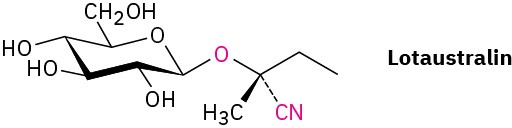
Problem 20-28
2-Bromo-6,6-dimethylcyclohexanone gives 2,2-dimethylcyclopentanecarboxylic acid on treatment with aqueous NaOH followed by acidification, a process called the Favorskii reaction. Propose a mechanism.
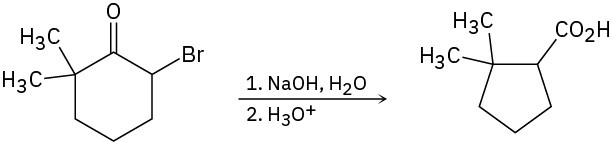
Problem 20-29
Naturally occurring compounds called terpenoids, which we’ll discuss in Section 27.5, are biosynthesized by a pathway that involves loss of CO2 from 3-phosphomevalonate 5- diphosphate to yield isopentenyl diphosphate. Use curved arrows to show the mechanism of this reaction.
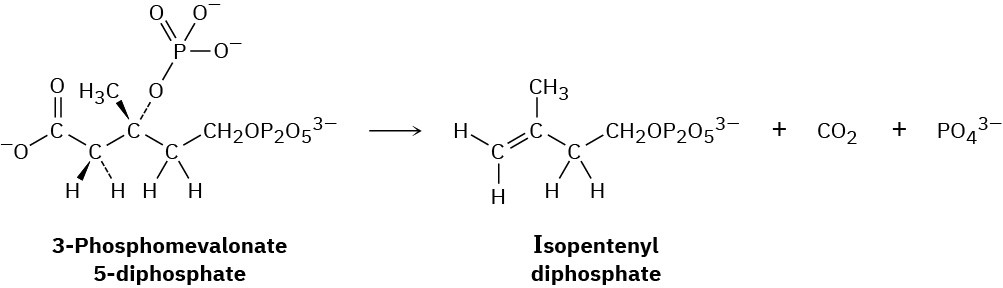
Problem 20-30
In the Ritter reaction, an alkene reacts with a nitrile in the presence of strong aqueous sulfuric acid to yield an amide. Propose a mechanism.
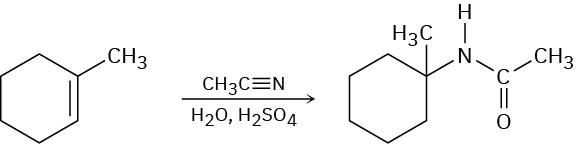
Naming Carboxylic Acids and Nitriles
Problem 20-31
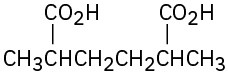 Give IUPAC names for the following compounds: (a)
Give IUPAC names for the following compounds: (a)
(b)
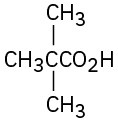
(c)
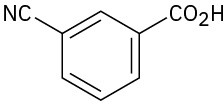
(d)
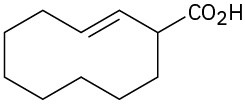
(e)
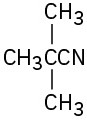
(f)
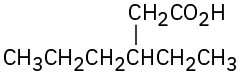
(g)
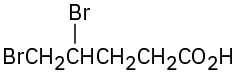
(h)
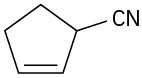
Problem 20-32
Draw structures corresponding to the following IUPAC names: (a)
cis-1,2-Cyclohexanedicarboxylic acid (b)
Heptanedioic acid (c)
2-Hexen-4-ynoic acid (d)
4-Ethyl-2-propyloctanoic acid (e)
3-Chlorophthalic acid (f)
Triphenylacetic acid (g)
2-Cyclobutenecarbonitrile (h)
m-Benzoylbenzonitrile Problem 20-33
Draw and name the following compounds:
(a)
The eight carboxylic acids with the formula C6H12O2 (b)
Three nitriles with the formula C5H7N Problem 20-34
Pregabalin, marketed as Lyrica, is an anticonvulsant drug that is also effective in treating chronic pain. The IUPAC name of pregabalin is (S)-3-(aminomethyl)-5-methylhexanoic acid. (An aminomethyl group is –CH2NH2.) Draw the structure of pregabalin.
Problem 20-35
Isocitric acid, an intermediate in the citric acid cycle of food metabolism, has the systematic name (2R,3S)-3-carboxy-2-hydroxypentanedioic acid. Draw the structure.
Acidity of Carboxylic Acids
Problem 20-36
Order the compounds in each of the following sets with respect to increasing acidity: (a)
Acetic acid, oxalic acid, formic acid (b)
p-Bromobenzoic acid, p-nitrobenzoic acid, 2,4-dinitrobenzoic acid (c)
Fluoroacetic acid, 3-fluoropropanoic acid, iodoacetic acid Problem 20-37
Arrange the compounds in each of the following sets in order of increasing basicity: (a)
Magnesium acetate, magnesium hydroxide, methylmagnesium bromide (b)
Sodium benzoate, sodium p-nitrobenzoate, sodium acetylide (c)
Lithium hydroxide, lithium ethoxide, lithium formate Problem 20-38
Calculate the pKa’s of the following acids:
(a)
Lactic acid, Ka = 8.4 × 10–4 (b)
Acrylic acid, Ka = 5.6 × 10–6 Problem 20-39
Calculate the Ka’s of the following acids:
(a)
Citric acid, pKa = 3.14 (b)
Tartaric acid, pKa = 2.98 Problem 20-40
Thioglycolic acid, HSCH2CO2H, a substance used in depilatory agents (hair removers) has pKa = 3.42. What is the percent dissociation of thioglycolic acid in a buffer solution at pH = 3.0?
Problem 20-41
In humans, the final product of purine degradation from DNA is uric acid, pKa = 5.61, which is excreted in the urine. What is the percent dissociation of uric acid in urine at a typical pH
= 6.0? Why do you think uric acid is acidic even though it does not have a CO2H group?
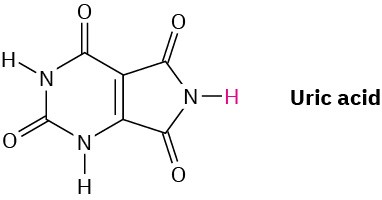
Problem 20-42
Some pKa data for simple dibasic acids is shown. How can you account for the fact that the difference between the first and second ionization constants decreases with increasing distance between the carboxyl groups?
|
Name |
Structure |
pK1 |
pK2 |
|
Oxalic |
HO2CCO2H |
1.2 |
4.2 |
|
Succinic |
HO2CCH2CH2CO2H |
4.2 |
5.6 |
|
Adipic |
HO2C(CH2)4CO2H |
4.4 |
5.4 |
Reactions of Carboxylic Acids and Nitriles
Problem 20-43
How could you convert butanoic acid into the following compounds? Write each step showing the reagents needed.
(a)
1-Butanol (b)
1-Bromobutane (c)
Pentanoic acid (d)
1-Butene (e) Octane
Problem 20-44
How could you convert each of the following compounds into butanoic acid? Write each step showing all reagents.
(a)
1-Butanol (b)
1-Bromobutane (c)
1-Butene (d)
1-Bromopropane (e)
4-Octene Problem 20-45
How could you convert butanenitrile into the following compounds? Write each step showing the reagents needed.
(a)
- Butanol (b) Butylamine (c)
- Methyl-3-hexanone Problem 20-46
How would you prepare the following compounds from benzene? More than one step is needed in each case.
(a)
m-Chlorobenzoic acid (b)
p-Bromobenzoic acid (c)
Phenylacetic acid, C6H5CH2CO2H Problem 20-47
Predict the product of the reaction of p-methylbenzoic acid with each of the following: (a)
LiAlH4, then H3O+ (b)
N-Bromosuccinimide in CCl4 (c)
CH3MgBr in ether, then H3O+ (d)
KMnO4, H3O+
Problem 20-48
Using 13CO2 as your only source of labeled carbon, along with any other compounds needed, how would you synthesize the following compounds?
(a) CH3CH213CO2H
(b) PRODCH313CH2CO2H
Problem 20-49
How would you carry out the following transformations?
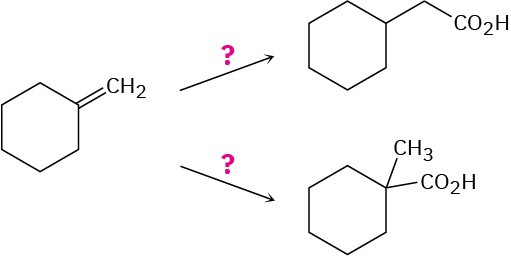
Problem 20-50
Which method—Grignard carboxylation or nitrile hydrolysis—would you use for each of the following reactions? Explain.
(a)
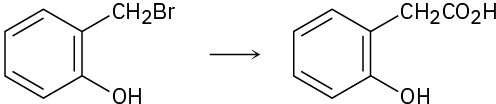
(b)
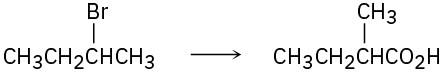
(c)
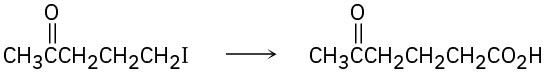
(d)

Problem 20-51
1,6-Hexanediamine, a starting material needed for making nylon, can be made from 1,3- butadiene. How would you accomplish the synthesis?
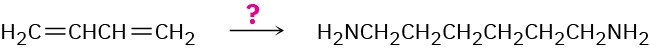
Problem 20-52
- Methyl-2-hexenoic acid (mixture of E and Z isomers) has been identified as the substance responsible for the odor of human sweat. Synthesize the compound from starting materials having five or fewer carbons.
Spectroscopy
Problem 20-53
Propose a structure for a compound C6H12O2 that dissolves in dilute NaOH and shows the following 1H NMR spectrum: 1.08 δ (9 H, singlet), 2.2 δ (2 H, singlet), and 11.2 δ (1 H, singlet).
Problem 20-54
What spectroscopic method could you use to distinguish among the following three isomeric acids? Tell what characteristic features you would expect for each acid.
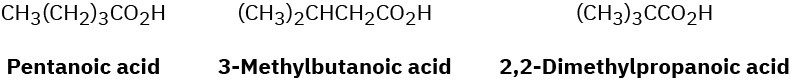
Problem 20-55
How would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers?
(a)
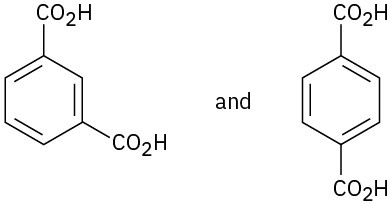
(b)
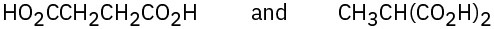
(c)

(d)
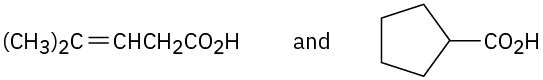
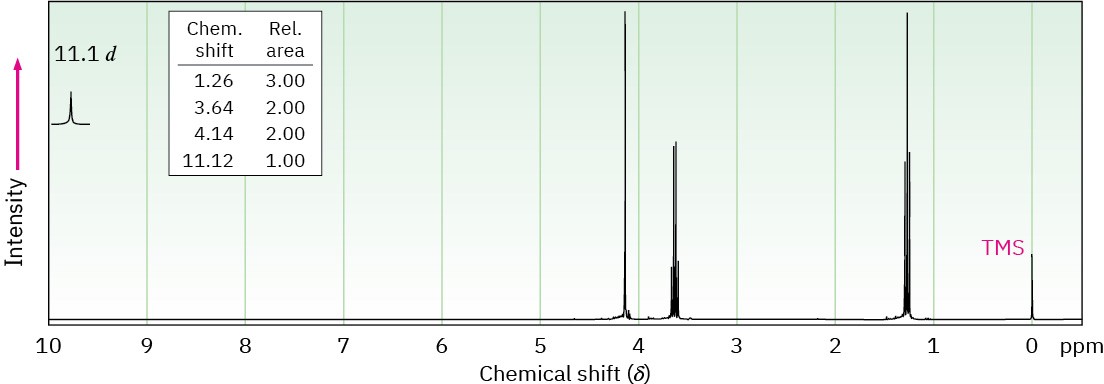
Problem 20-56
Compound A, C4H8O3, has infrared absorptions at 1710 and 2500 to 3100 cm–1 and has the
1H NMR spectrum shown. Propose a structure for A.
General Problems
Problem 20-57
A chemist in need of 2,2-dimethylpentanoic acid decided to synthesize some by reaction of 2-chloro-2-methylpentane with NaCN, followed by hydrolysis of the product. After the reaction sequence was carried out, however, none of the desired product could be found. What do you suppose went wrong?
Problem 20-58
Show how you might prepare the anti-inflammatory agent ibuprofen starting from isobutylbenzene. More than one step is needed.
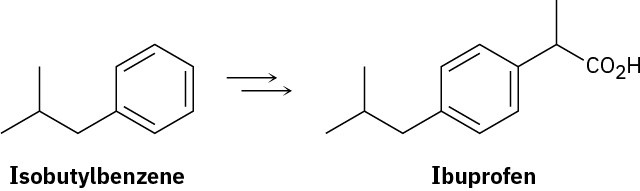
Problem 20-59
The following synthetic schemes all have at least one flaw in them. What is wrong with each?
(a)
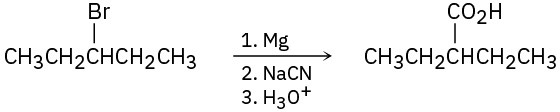
(b)
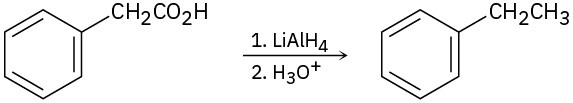
(c)
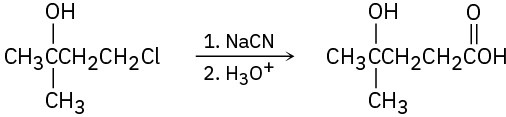
Problem 20-60
p-Aminobenzoic acid (PABA) was once widely used as a sunscreen agent. Propose a synthesis of PABA starting from toluene.
Problem 20-61
Propose a synthesis of the anti-inflammatory drug fenclorac from phenylcyclohexane.
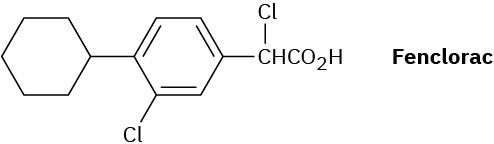
Problem 20-62
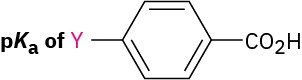
The pKa’s of five p-substituted benzoic acids (YC6H4CO2H) are listed below. Rank the corresponding substituted benzenes (YC6H5) in order of their increasing reactivity toward electrophilic aromatic substitution. If benzoic acid has pKa = 4.19, which of the substituents are activators and which are deactivators?
|
Substituent Y |
|
|
–Si(CH3)3 |
4.27 |
|
–CH═CHC≡N |
4.03 |
|
–HgCH3 |
4.10 |
|
–OSO2CH3 |
3.84 |
|
–PCl2 |
3.59 |
Problem 20-63
How would you carry out the following transformations? More than one step is needed in each case.
(a)
(b)
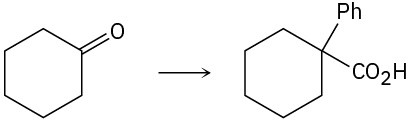
Problem 20-64
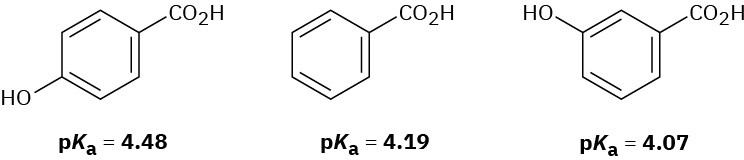
The following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.
Problem 20-65
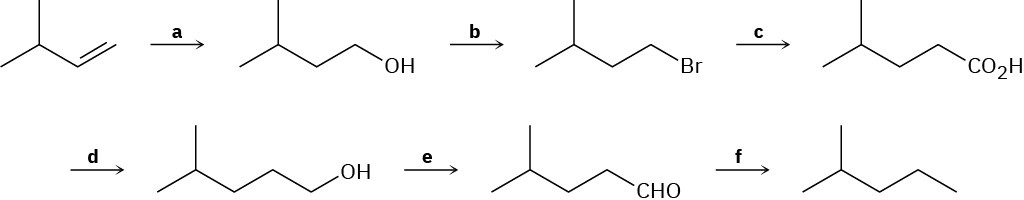
Identify the missing reagents a–f in the following scheme:
Problem 20-66
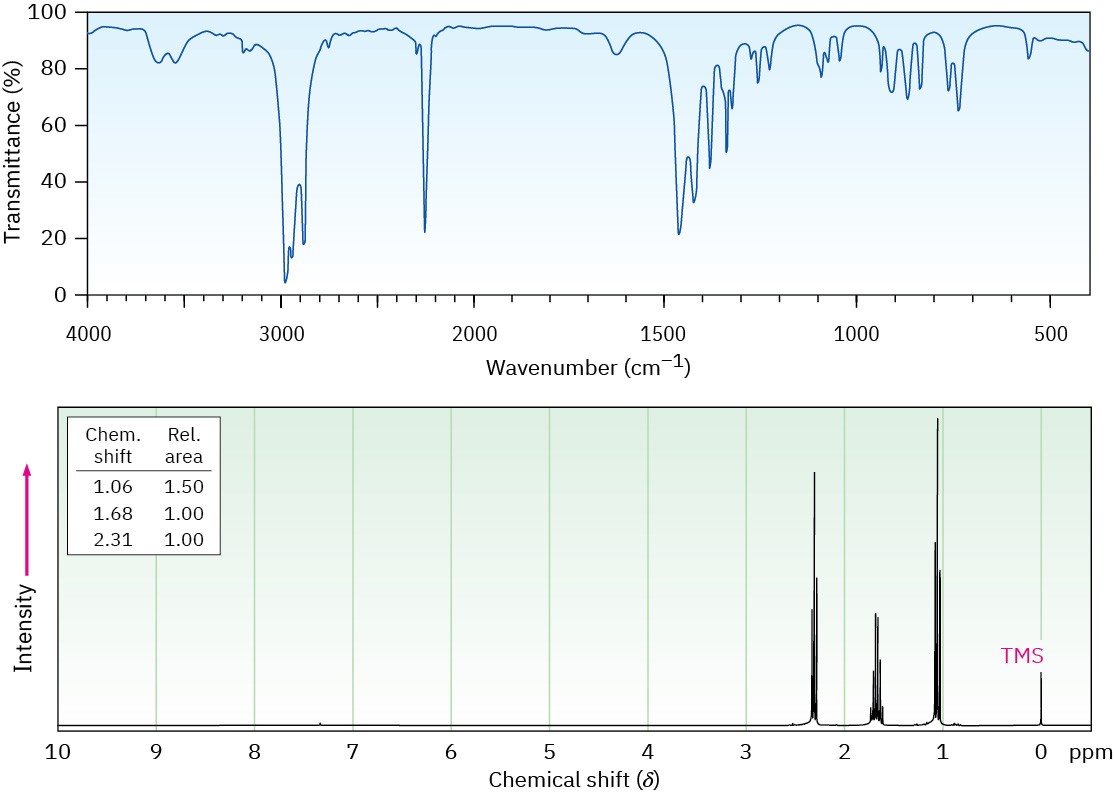
Propose a structure for a compound, C4H7N, that has the following IR and 1H NMR spectra:
Problem 20-67
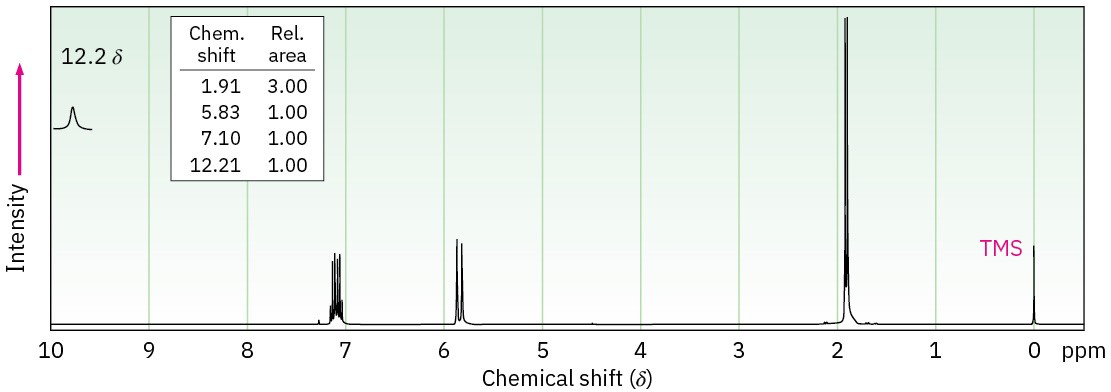
The two 1H NMR spectra shown here belong to crotonic acid (trans-CH3CH=CHCO2H) and methacrylic acid [H2C=C(CH3)CO2H]. Which spectrum corresponds to which acid? Explain.
(a)
(b)
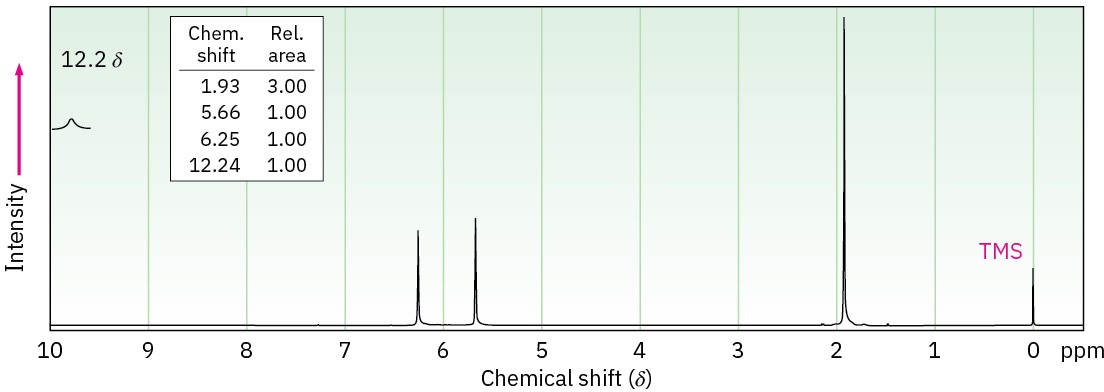
Problem 20-68
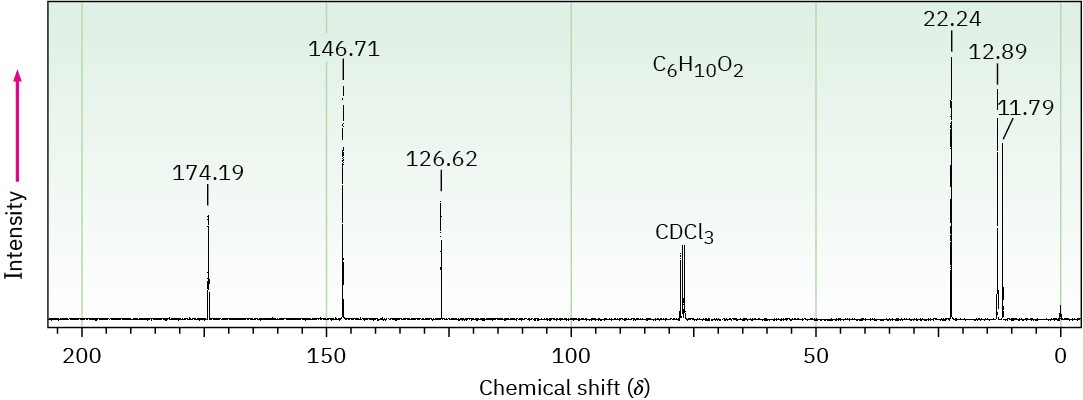
The 1H and 13C NMR spectra below belong to a compound with formula C6H10O2. Propose a structure for this compound.
(a)
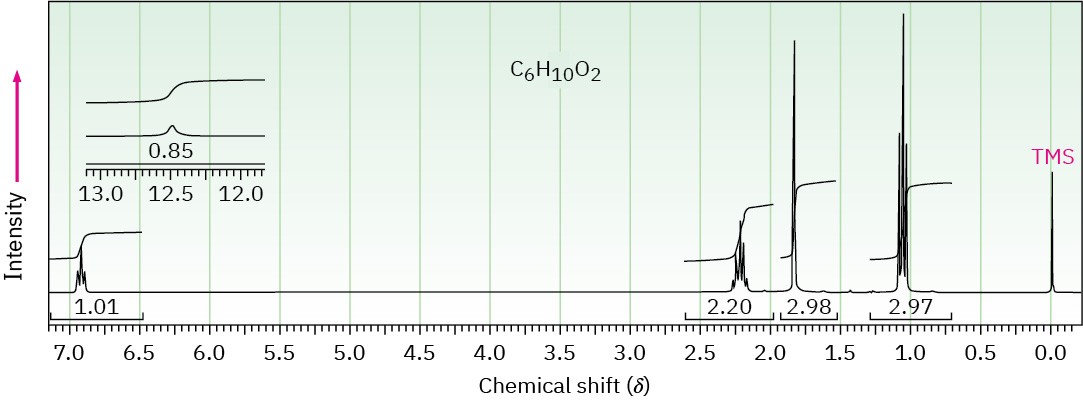
(b)
Problem 20-69
Propose structures for carboxylic acids that show the following peaks in their 13C NMR spectra. Assume that the kinds of carbons (1°, 2°, 3°, or 4°) have been assigned by DEPT- NMR.
(a)
C7H12O2: 25.5 δ (2°), 25.9 δ (2°), 29.0 δ (2°), 43.1 δ (3°), 183.0 δ (4°)
(b)
C8H8O2: 21.4 δ (1°), 128.3 δ (4°), 129.0 δ (3°), 129.7 δ (3°), 143.1 δ (4°), 168.2 δ (4°)
Problem 20-70
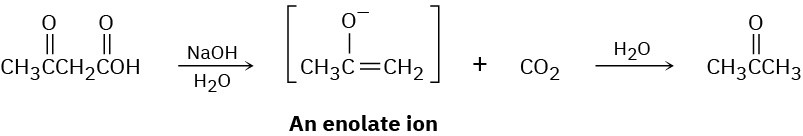
Carboxylic acids having a second carbonyl group two atoms away lose CO2 (decarboxylate) through an intermediate enolate ion when treated with base. Write the mechanism of this decarboxylation reaction using curved arrows to show the electron flow in each step.