25.4 Configurations of the Aldoses
An aldotetrose is a four-carbon sugar with two chirality centers. Thus, there are 22 = 4 possible stereoisomeric aldotetroses, or two D,L pairs of enantiomers named erythrose and threose.
Aldopentoses have three chirality centers and a total of 23 = 8 possible stereoisomers, or four D,L pairs of enantiomers. These four pairs are called ribose, arabinose, xylose, and lyxose. All except lyxose occur widely in nature. D-Ribose is an important constituent of RNA (ribonucleic acid), L-arabinose is found in many plants, and D-xylose is found in wood.
Aldohexoses have four chirality centers and a total of 24 = 16 possible stereoisomers, or eight D,L pairs of enantiomers. The names of the eight are allose, altrose, glucose, mannose, gulose, idose, galactose, and talose. Only D-glucose, from starch and cellulose, and D- galactose, from gums and fruit pectins, are widely distributed in nature. D-mannose and D- talose also occur naturally but in lesser abundance.
Fischer projections of the four-, five-, and six-carbon D aldoses are shown in Figure 25.4. Starting with D-glyceraldehyde, we can imagine constructing the two D aldotetroses by inserting a new chirality center just below the aldehyde carbon. Each of the two D aldotetroses then leads to two D aldopentoses (four total), and each of the four D aldopentoses leads to two D aldohexoses (eight total). In addition, each of the D aldoses in Figure 25.4 has an L enantiomer, which is not shown.
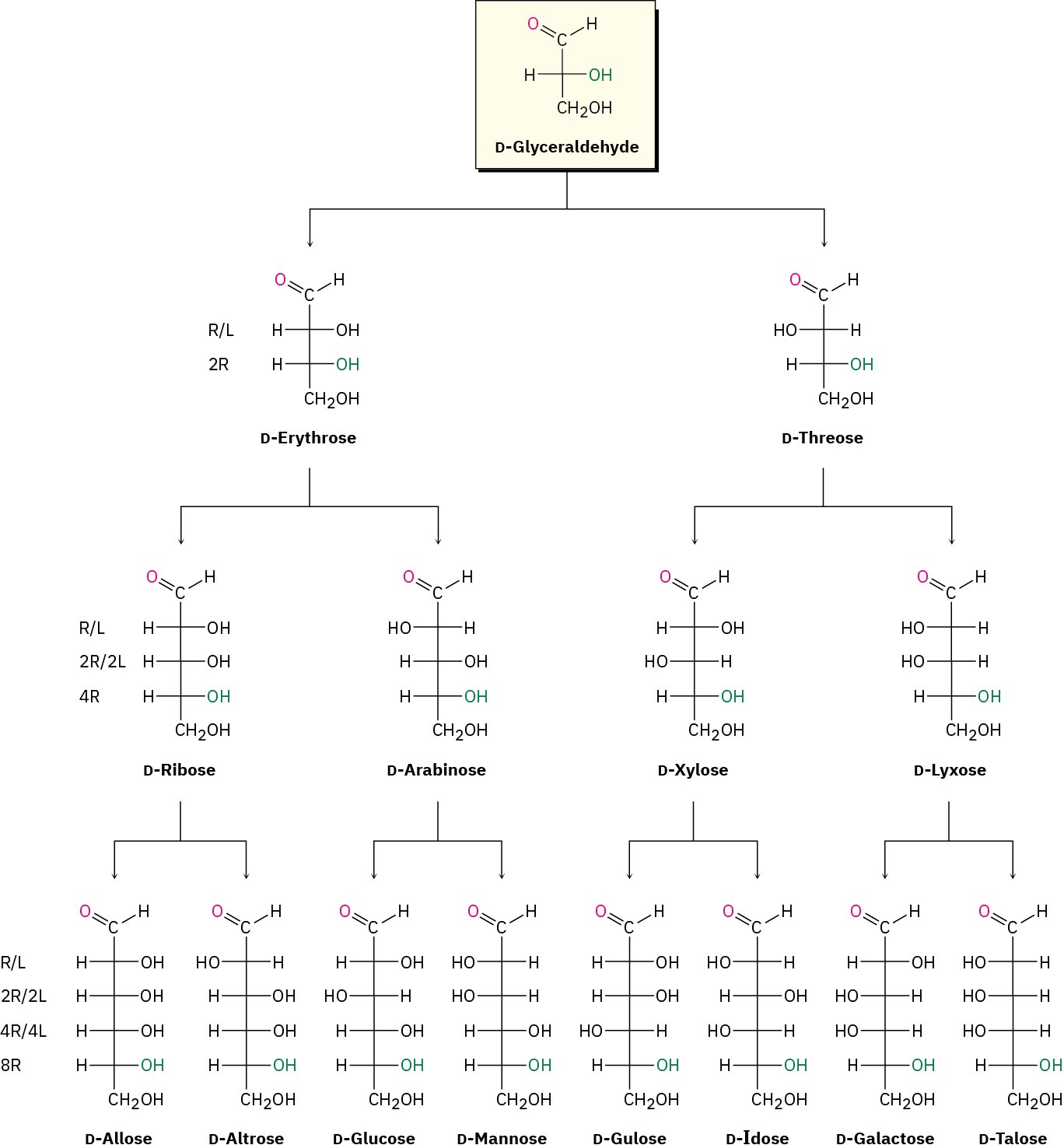
Figure 25.4 Configurations of D aldoses. The structures are arranged from left to right so that the –OH groups on C2 alternate right/left (R/L) across each series. Similarly, the – OH groups at C3 alternate two right/two left (2R/2L), the –OH groups at C4 alternate 4R/4L, and the –OH groups at C5 are to the right in all eight (8R). Each D aldose has a corresponding L enantiomer that is not shown.
Louis Fieser of Harvard University long ago suggested a simple procedure for remembering the names and structures of the eight D aldohexoses:
STEP 1
Set up eight Fischer projections with the –CHO group on top and the –CH2OH group at the bottom.
STEP 2
At C5, place all eight –OH groups to the right (D series).
STEP 3
At C4, alternate four –OH groups to the right and four to the left.
STEP 4
At C3, alternate two –OH groups to the right, two to the left.
STEP 5
At C2, alternate –OH groups right, left, right, left.
STEP 6
Name the eight isomers using the mnemonic “All altruists gladly make gum in gallon tanks.”
The structures of the four D aldopentoses can be generated in a similar way and named by the mnemonic suggested by a Cornell University undergraduate: “RIBs ARe eXtra Lean.”
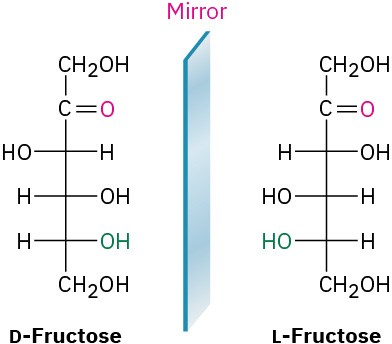
Worked Example 25.2Drawing a Fischer ProjectionDraw a Fischer projection of L-fructose.StrategyBecause L-fructose is the enantiomer of D-fructose, simply look at the structure of D- fructose and reverse the configuration at each chirality center.Solution
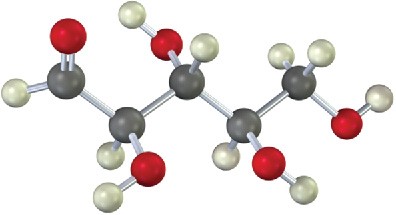
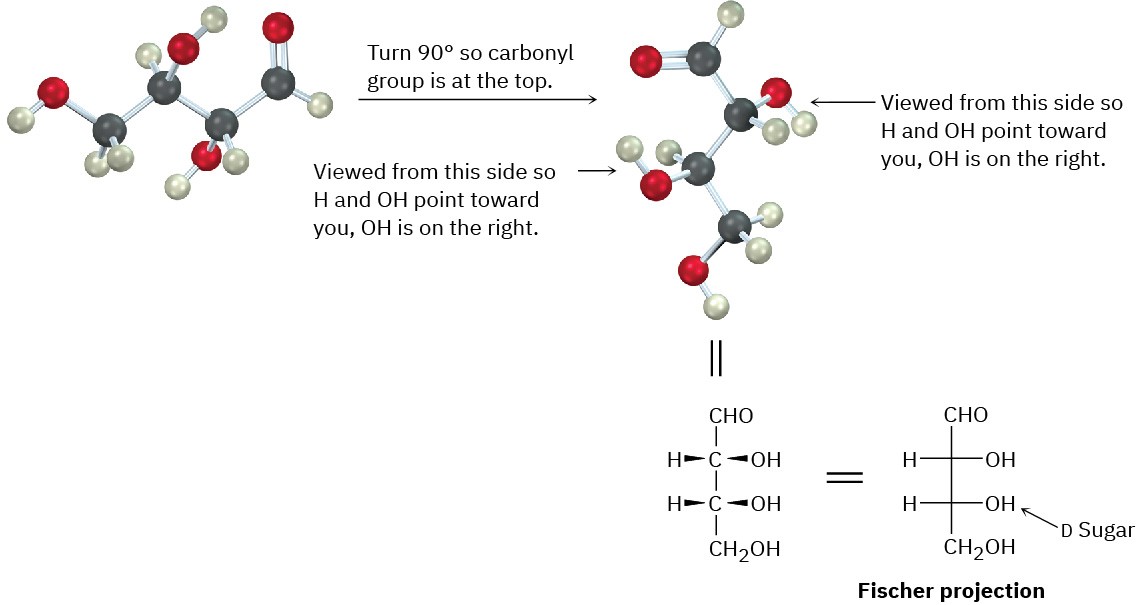
Worked Example 25.3Drawing a Fischer Projection of a Molecular ModelDraw the following aldotetrose as a Fischer projection, and identify it as a D sugar or an L sugar:
StrategyThe Fischer projection of a monosaccharide is drawn vertically, with the carbonyl group at or near the top and the –CH2OH group at the bottom. The interior –H and –OH are drawn to the sides, pointing out of the page toward the viewer.Solution
Problem 25-8
Only the D sugars are shown in Figure 25.4. Draw Fischer projections for the following L sugars:
(a)
L-Xylose (b)
L-Galactose (c)
L-Allose Problem 25-9
How many aldoheptoses are there? How many are D sugars, and how many are L sugars?
Problem 25-10
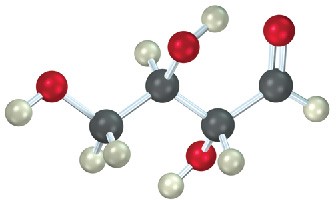
The following model is that of an aldopentose. Draw a Fischer projection of the sugar, name it, and identify it as a D sugar or an L sugar.