23.13 Some Biological Carbonyl Condensation Reactions
Biological Aldol Reactions
Aldol reactions occur in many biological pathways but are particularly common in carbohydrate metabolism, where enzymes called aldolases catalyze the addition of a ketone enolate ion to an aldehyde. Aldolases occur in all organisms and are of two types. Type I aldolases occur primarily in animals and higher plants; type II aldolases occur primarily in fungi and bacteria. Both types catalyze the same kind of reaction, but type I aldolases operate through an enamine while type II aldolases require a metal ion (usually Zn2+) as a Lewis acid and operate through an enolate ion.
An example of an aldolase-catalyzed reaction occurs in glucose biosynthesis when dihydroxyacetone phosphate reacts with glyceraldehyde 3-phosphate to give fructose 1,6- bisphosphate. In animals and higher plants, dihydroxyacetone phosphate is first converted into an enamine by reaction with the –NH2 group on a lysine amino acid in the enzyme. The enamine then adds to glyceraldehyde 3-phosphate, and the iminium ion that results is hydrolyzed. In bacteria and fungi, the aldol reaction occurs directly, with the ketone carbonyl group of glyceraldehyde 3-phosphate complexed to a Zn2+ ion to make it a better acceptor.
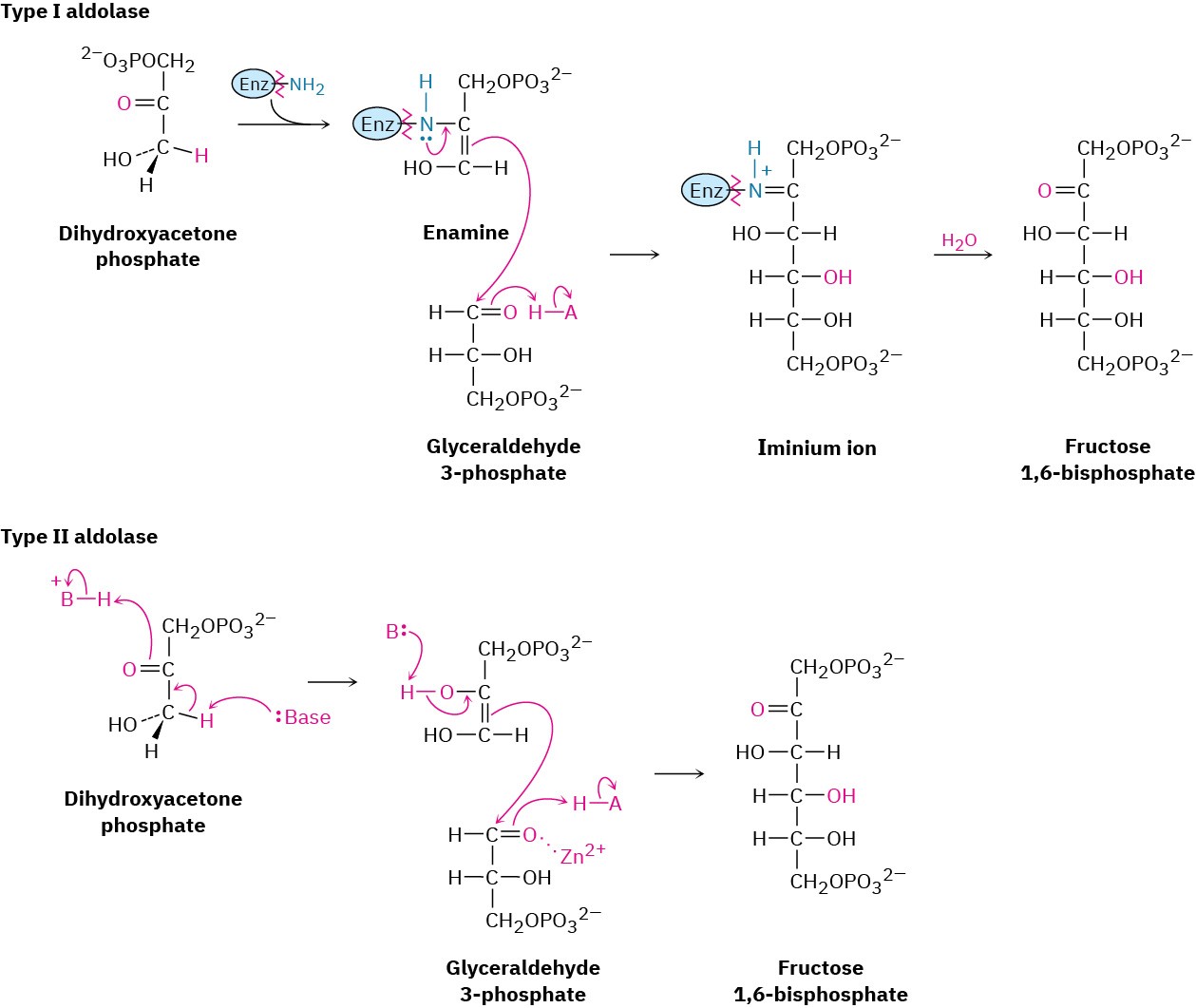
Note that the aldolase-catalyzed reactions are mixed aldol reactions, which take place between two different partners, as opposed to the symmetrical aldol reactions between identical partners usually carried out in the laboratory. Mixed aldol reactions often give mixtures of products in the laboratory but are successful in living systems because of the selectivity of the enzyme catalysts.
Biological Claisen Condensations
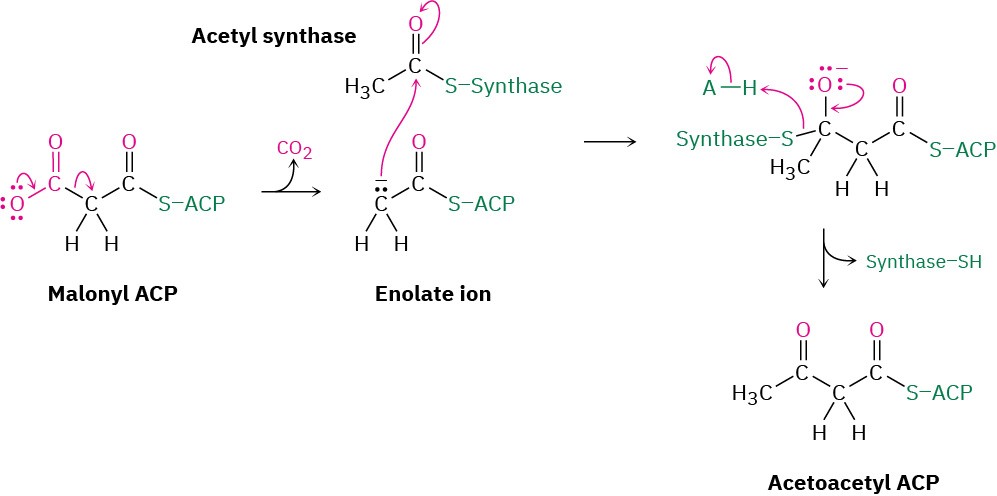
Claisen condensations, like aldol reactions, also occur in a large number of biological pathways. In fatty-acid biosynthesis, for instance, an enolate ion generated by decarboxylation (Section 22.7) of malonyl ACP adds to the carbonyl group of another acyl group bonded through a thioester linkage to a synthase enzyme. The tetrahedral intermediate that results then expels the synthase, giving acetoacetyl ACP. (The abbreviation ACP stands for acyl carrier protein, which forms thioester bonds to acyl groups.)
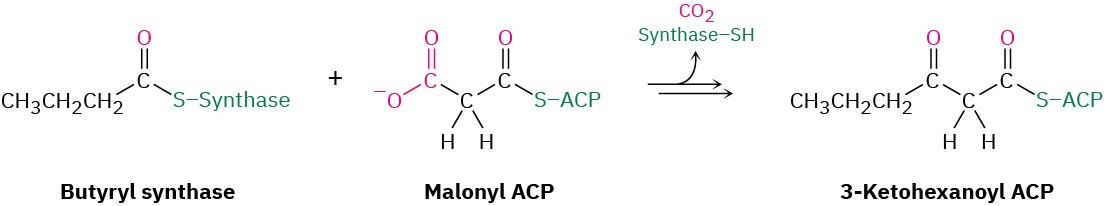
Mixed Claisen condensations also occur frequently in living organisms, particularly in the pathway for fatty-acid biosynthesis that we’ll discuss in Section 29.4. Butyryl synthase, for instance, reacts with malonyl ACP in a mixed Claisen condensation to give 3-ketohexanoyl ACP.
Additional Problems 23 • Additional Problems 23 • Additional Problems Visualizing Chemistry Problem 23-23
What ketones or aldehydes might the following enones have been prepared from by aldol reaction?
(a)
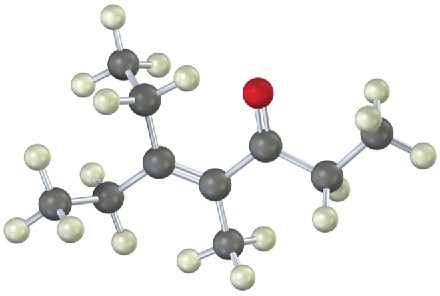
(b)
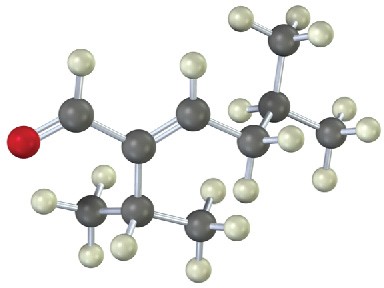
Problem 23-24
The following structure represents an intermediate formed by addition of an ester enolate ion to a second ester molecule. Identify the reactant, the leaving group, and the product.
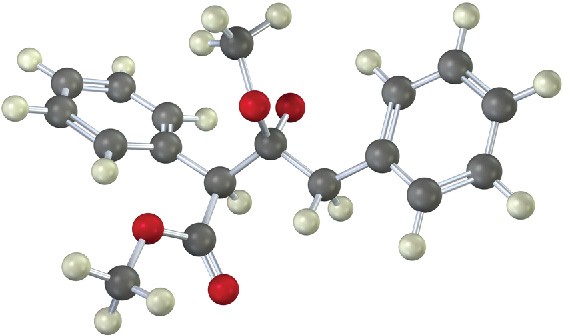
Problem 23-25
The following molecule was formed by an intramolecular aldol reaction. What dicarbonyl precursor was used for its preparation?
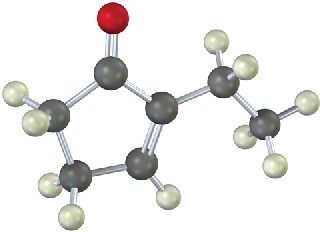
Problem 23-26
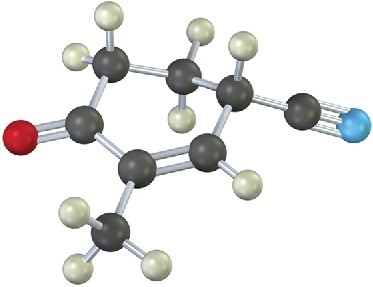
The following molecule was formed by a Robinson annulation reaction. What reactants were used?
Mechanism Problems
Problem 23-27
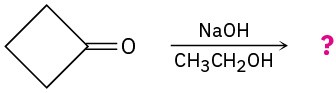
Predict the addition product for each of the following reactions and write the mechanism. (a)
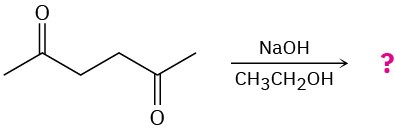
(b)
Problem 23-28
Based on your answers to Problem 23-27, predict the dehydration product for both reactions and write the mechanism.
Problem 23-29
Predict the product(s) and provide the mechanisms for the following reactions. (a)
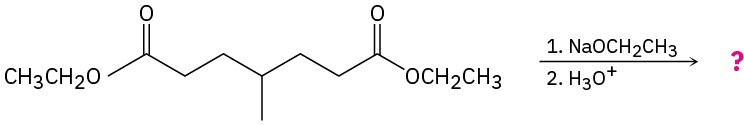
(b)
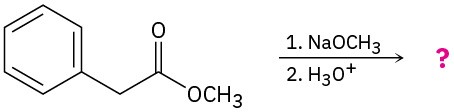
Problem 23-30
Predict the product(s) and provide the mechanisms for the following reactions. (a)
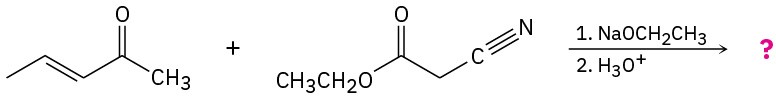
(b)
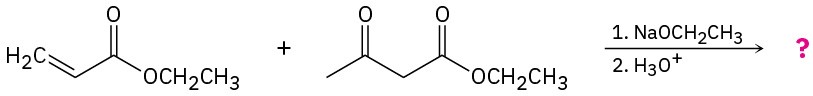
Problem 23-31
Predict the product(s) and provide the mechanisms for the following reactions. (a)
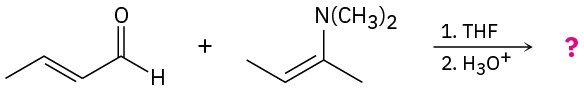
(b)
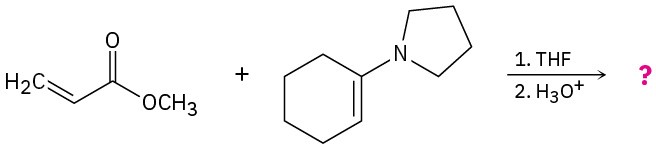
Problem 23-32
Knoevenagel condensation is a reaction involving an active methylene compound (a CH2 flanked by two electron-withdrawing groups) and an aldehyde and ketone. Propose a mechanism for the reaction below.
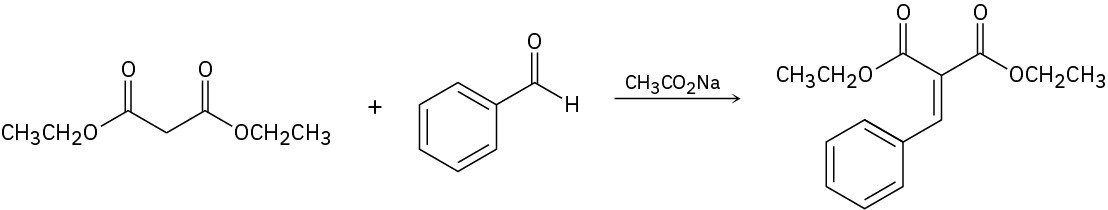
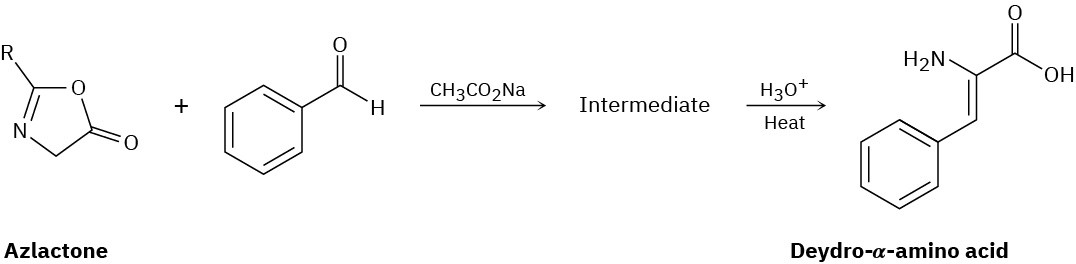
Problem 23-33
Azlactones are important starting materials used in the synthesis of dehydro α-aminoacids. They react with aldehydes to form an intermediate that is hydrolyzed under acidic conditions to give the final amino acid product. Provide the structure of the intermediate and propose a mechanism for its formation.
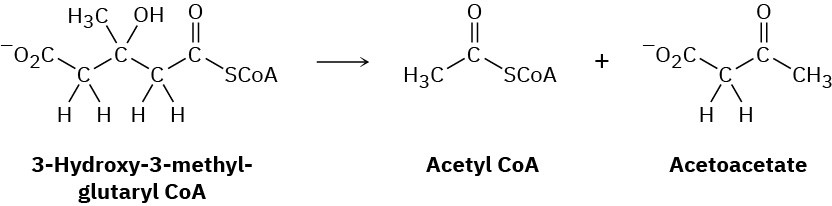
Problem 23-34
Leucine, one of the twenty amino acids found in proteins, is metabolized by a pathway that includes the following step. Propose a mechanism.
Problem 23-35
Isoleucine, another of the twenty amino acids found in proteins, is metabolized by a pathway that includes the following step. Propose a mechanism.
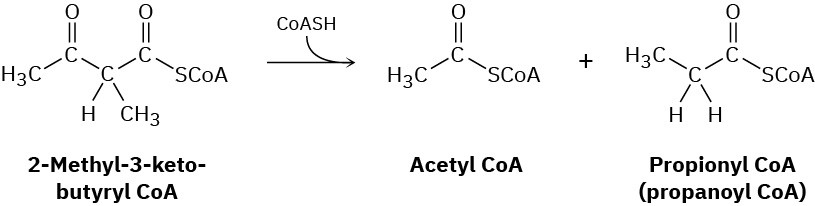
Problem 23-36
The first step in the citric acid cycle of food metabolism is reaction of oxaloacetate with acetyl CoA to give citrate. Propose a mechanism, using acid or base catalysis as needed.
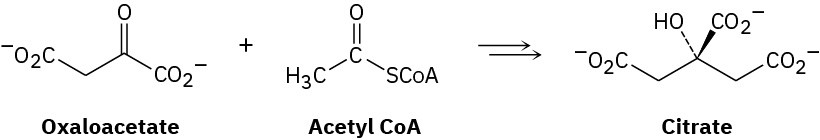
Problem 23-37
The amino acid leucine is biosynthesized from α-ketoisovalerate by the following sequence of steps. Show the mechanism of each.
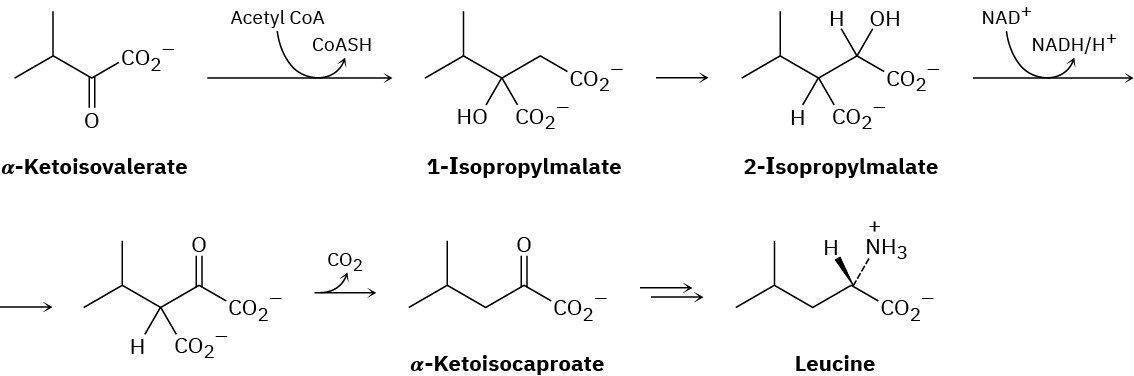
Problem 23-38
The Knoevenagel reaction was introduced in Problem 23-2. Show the mechanism for the Knoevenagel reaction of diethyl malonate with benzaldehyde.
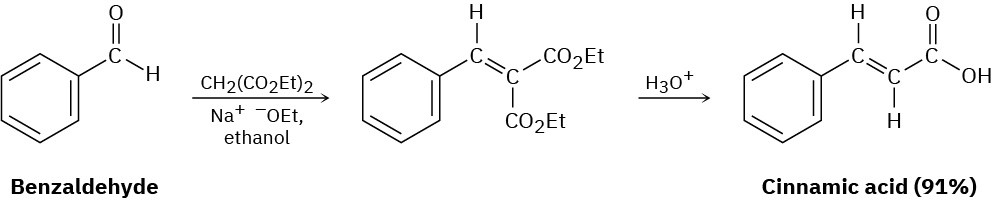
Problem 23-39
The Darzens reaction involves a two-step, base-catalyzed condensation of ethyl chloroacetate with a ketone to yield an epoxy ester. The first step is a carbonyl condensation reaction, and the second step is an SN2 reaction. Write both steps, and show their mechanisms.
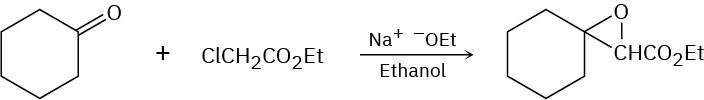
Problem 23-40
The following reaction involves a hydrolysis followed by an intramolecular nucleophilic acyl substitution reaction. Write both steps, and show their mechanisms.
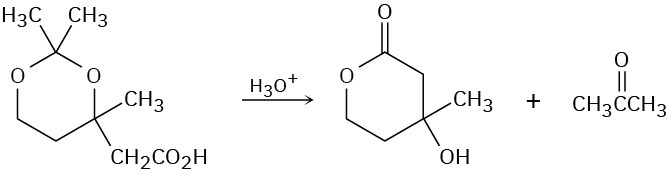
Problem 23-41
The following reaction involves an intramolecular Michael reaction followed by an intramolecular aldol reaction. Write both steps, and show their mechanisms.
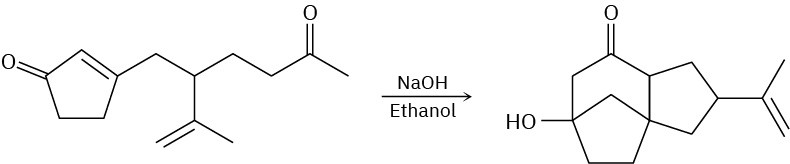
Problem 23-42
The following reaction involves a conjugate addition reaction followed by an intramolecular Claisen condensation. Write both steps, and show their mechanisms.
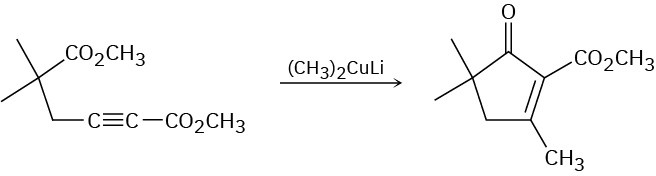
Problem 23-43
The following reaction involves an intramolecular aldol reaction followed by a retro aldol- like reaction. Write both steps, and show their mechanisms.
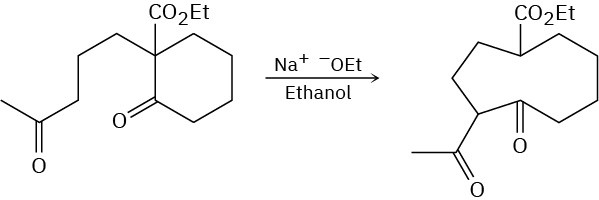
Problem 23-44
Propose a mechanism for the following base-catalyzed isomerization:
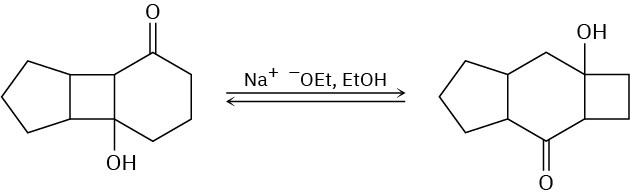
Problem 23-45
The Mannich reaction of a ketone, an amine, and an aldehyde is one of the few three- component reactions in organic chemistry. Cyclohexanone, for example, reacts with dimethylamine and acetaldehyde to yield an amino ketone. The reaction takes place in two steps, both of which are typical carbonyl-group reactions.
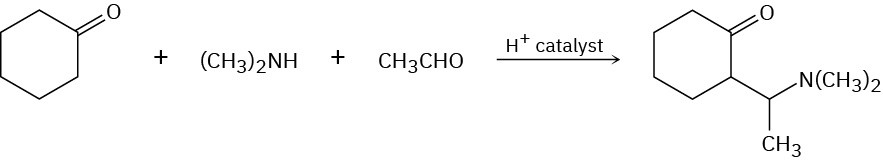
(a)
The first step is reaction between the aldehyde and the amine to yield an intermediate iminium ion (R2C═NR2+) plus water. Propose a mechanism, and show the structure of the intermediate iminium ion.
(b)
The second step is reaction between the iminium ion intermediate and the ketone to yield the final product. Propose a mechanism.
Problem 23-46
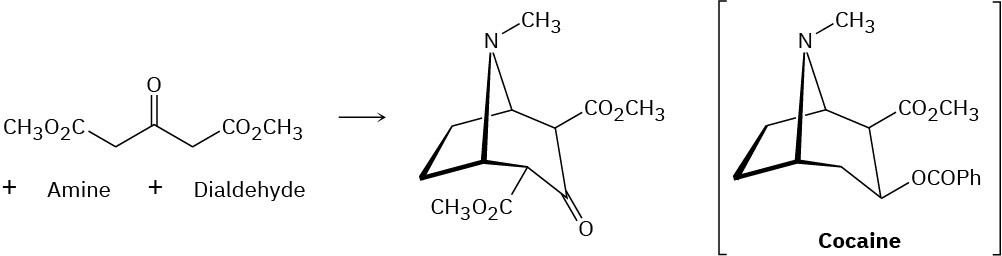
Cocaine has been prepared by a sequence beginning with a Mannich reaction (Problem 23- 45) between dimethyl acetonedicarboxylate, an amine, and a dialdehyde. Show the structures of the amine and dialdehyde.
Problem 23-47
Propose a mechanism to account for the following reaction of an enamine with an alkyl halide:
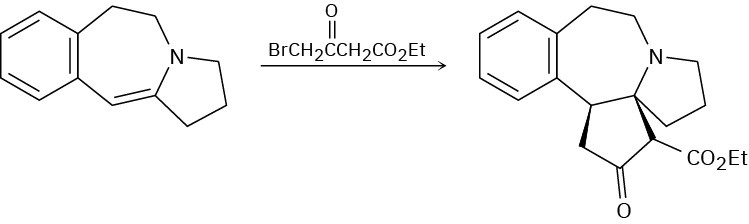
Aldol Reactions
Problem 23-48
Which of the following compounds would you expect to undergo aldol self-condensation? Show the product of each successful reaction.
(a) Trimethylacetaldehyde(b) Cyclobutanone(c) Benzophenone (diphenyl ketone)
(d) 3-Pentanone(e) Decanal(f) 3-Phenyl-2-propenal Problem 23-49
How might you synthesize each of the following compounds using an aldol reaction? Show the structure of the starting aldehyde(s) or ketone(s) you would use in each case.
(a)
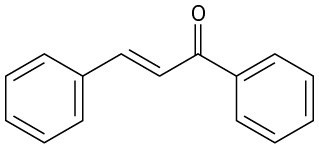
(b)
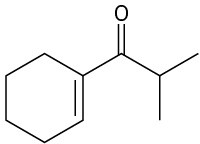
(c)
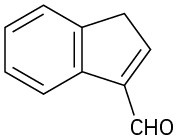
(d)
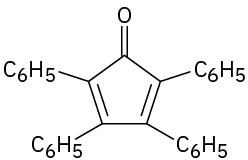
Problem 23-50
What product would you expect to obtain from aldol cyclization of hexanedial, OHCCH2CH2CH2CH2CHO?
Problem 23-51
Intramolecular aldol cyclization of 2,5-heptanedione with aqueous NaOH yields a mixture of two enone products in the approximate ratio 9 : 1. Write their structures, and show how each is formed.
Problem 23-52
The major product formed by intramolecular aldol cyclization of 2,5-heptanedione (Problem 23-51) has two singlet absorptions in the 1H NMR spectrum, at 1.65 δ and 1.90 δ, and has no absorptions in the range 3 to 10 δ. What is its structure?
Problem 23-53
Treatment of the minor product formed in the intramolecular aldol cyclization of 2,5- heptanedione (Problems 23-51 and 23-52) with aqueous NaOH converts it into the major product. Propose a mechanism to account for this base-catalyzed isomerization.
Problem 23-54
How can you account for the fact that 2,2,6-trimethylcyclohexanone yields no detectable aldol product even though it has an acidic α hydrogen?
Problem 23-55
The aldol reaction is catalyzed by acid as well as base. What is the reactive nucleophile in the acid-catalyzed aldol reaction? Propose a mechanism.
Problem 23-56
Cinnamaldehyde, the aromatic constituent of cinnamon oil, can be synthesized by a mixed aldol condensation. Show the starting materials you would use, and write the reaction.
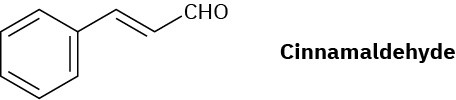
Problem 23-57
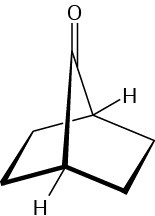
The following bicyclic ketone does not undergo aldol self-condensation even though it has two α hydrogen atoms. Explain.
Claisen Condensations
Problem 23-58
Give the structures of the possible Claisen condensation products from the following reactions. Tell which, if any, you would expect to predominate in each case.
(a)
CH3CO2Et + CH3CH2CO2Et
(b)
C6H5CO2Et + C6H5CH2CO2Et
(c)
EtOCO2Et + cyclohexanone (d)
C6H5CHO + CH3CO2Et
Problem 23-59
In the mixed Claisen reaction of cyclopentanone with ethyl formate, a much higher yield of the desired product is obtained by first mixing the two carbonyl components and then adding base, rather than by first mixing base with cyclopentanone and then adding ethyl formate. Explain.
Problem 23-60
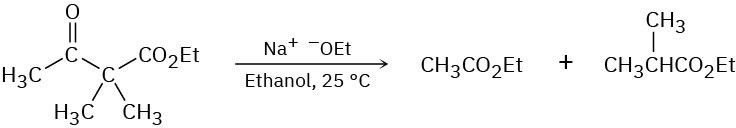
Ethyl dimethylacetoacetate reacts instantly at room temperature when treated with ethoxide ion to yield two products, ethyl acetate and ethyl 2-methylpropanoate. Propose a mechanism for this cleavage reaction.
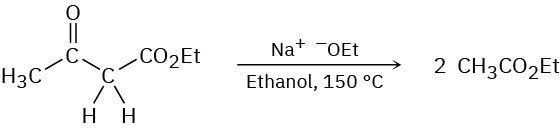
Problem 23-61
In contrast to the rapid reaction shown in Problem 23-60, ethyl acetoacetate requires a temperature over 150 °C to undergo the same kind of cleavage reaction. How can you explain the difference in reactivity?
Michael and Enamine Reactions
Problem 23-62
How might the following compounds be prepared using Michael reactions? Show the nucleophilic donor and the electrophilic acceptor in each case.
(a)
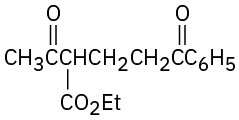
(b)
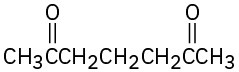
(c)
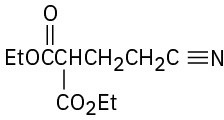
(d)
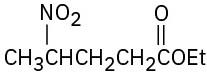
(e)
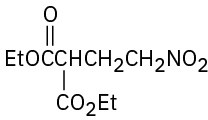
(f)
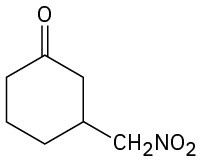
Problem 23-63
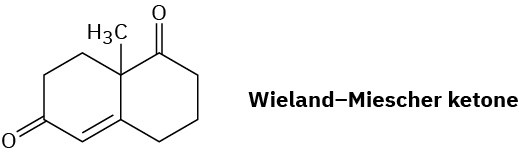
The so-called Wieland–Miescher ketone is a valuable starting material used in the synthesis of steroid hormones. How might you prepare it from 1,3-cyclohexanedione?
Problem 23-64
The Stork enamine reaction and the intramolecular aldol reaction can be carried out in sequence to allow the synthesis of cyclohexenones. For example, reaction of the pyrrolidine enamine of cyclohexanone with 3-buten-2-one, followed by enamine hydrolysis and base treatment, yields the product indicated. Write each step, and show the mechanism of each.
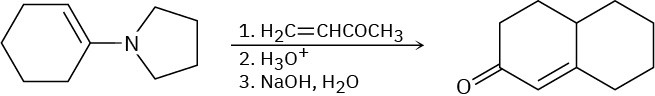
Problem 23-65
How could you prepare the following cyclohexenones by combining a Stork enamine reaction with an intramolecular aldol condensation? (See Problem 23-64.)
(a)
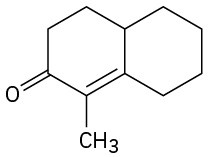
(b)
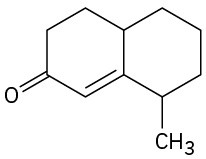
(c)
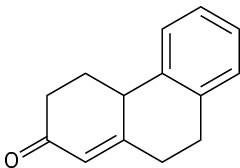
Problem 23-66
The following reaction involves two successive intramolecular Michael reactions. Write both steps, and show their mechanisms.
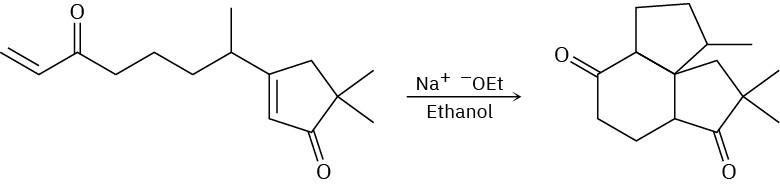
General Problems
Problem 23-67
What condensation products would you expect to obtain by treatment of the following substances with sodium ethoxide in ethanol?
(a)
Ethyl butanoate (b) Cycloheptanone (c)
3,7-Nonanedione (d)
- Phenylpropanal Problem 23-68
The following reactions are unlikely to provide the indicated product in high yield. What is wrong with each?
(a)
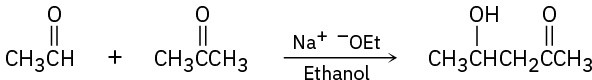
(b)
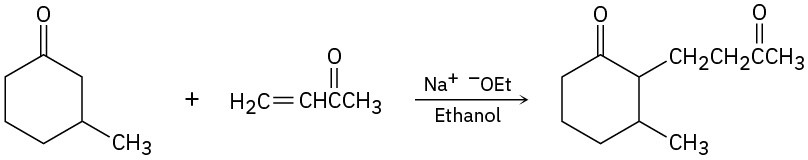
(c)
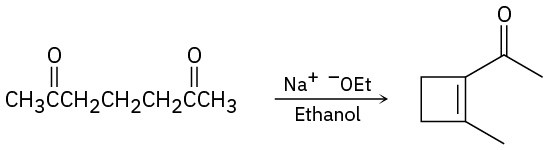
Problem 23-69
Fill in the missing reagents a–h in the following scheme:
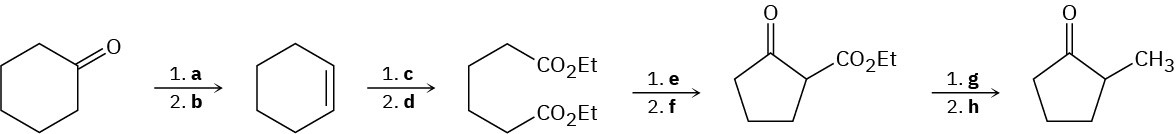
Problem 23-70
How would you prepare the following compounds from cyclohexanone? (a)
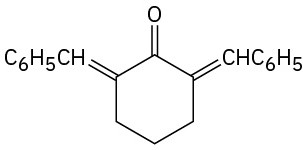
(b)
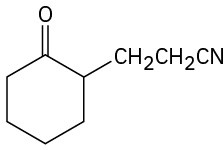
(c)
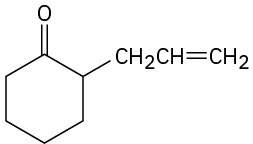
(d)
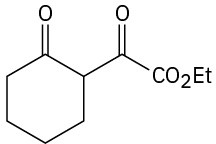
Problem 23-71
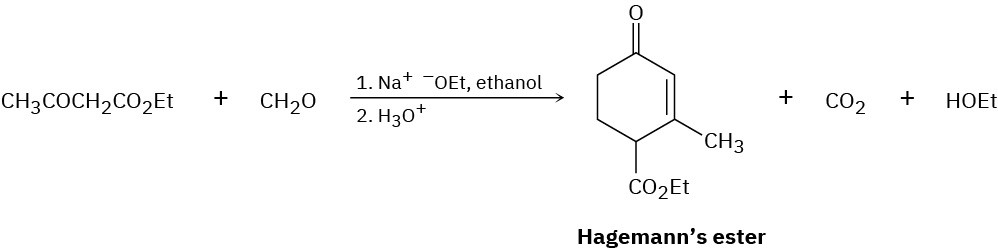
The compound known as Hagemann’s ester is prepared by treatment of a mixture of formaldehyde and ethyl acetoacetate with base, followed by acid-catalyzed decarboxylation.
(a)
The first step is an aldol-like condensation between ethyl acetoacetate and formaldehyde to yield an α,β-unsaturated product. Write the reaction, and show the structure of the product.
(b)
The second step is a Michael reaction between ethyl acetoacetate and the unsaturated product of the first step. Show the structure of the product.
Problem 23-72
The third and fourth steps in the synthesis of Hagemann’s ester from ethyl acetoacetate and formaldehyde (Problem 23-71) are an intramolecular aldol cyclization to yield a substituted cyclohexenone, and a decarboxylation reaction. Write both reactions, and show the products of each step.
Problem 23-73
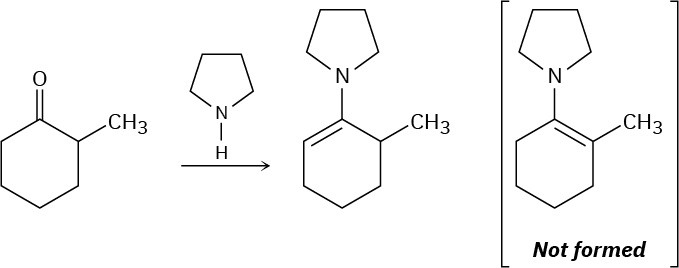
When 2-methylcyclohexanone is converted into an enamine, only one product is formed despite the fact that the starting ketone is unsymmetrical. Build molecular models of the two possible products and explain the fact that the sole product is the one with the double bond opposite the methyl-substituted carbon.