16.7 Benzyne
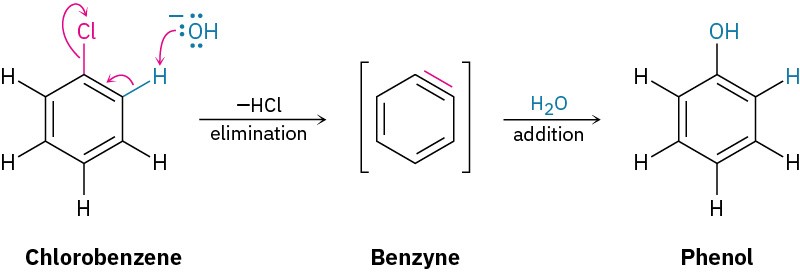
Halobenzenes without electron-withdrawing substituents don’t react with nucleophiles under most conditions. At high temperature and pressure, however, even chlorobenzene can be forced to react. Chemists at the Dow Chemical Company discovered in 1928 that phenol could be prepared on an industrial scale by treatment of chlorobenzene with dilute aqueous NaOH at 340 °C under 170 atm pressure.
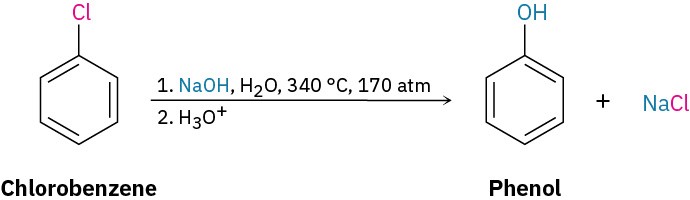
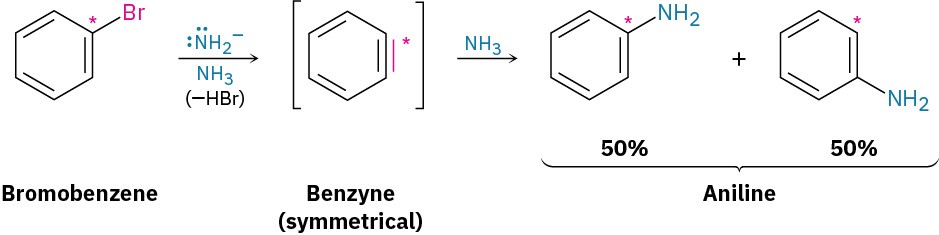
A similar substitution reaction occurs with other strong bases. Treatment of bromobenzene with potassium amide (KNH2) in liquid NH3 solvent, for instance, gives aniline. Curiously, though, when using bromobenzene labeled with radioactive 14C at the C1 position, the substitution product has equal amounts of the label at both C1 and C2, implying the presence of a symmetrical reaction intermediate in which C1 and C2 are equivalent.
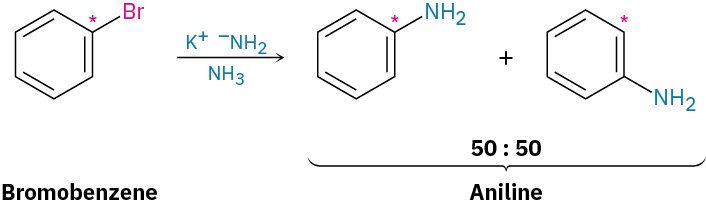
Further mechanistic evidence comes from trapping experiments. When bromobenzene is treated with KNH2 in the presence of a conjugated diene, such as furan, a Diels–Alder reaction (Section 14.4) occurs, implying that the symmetrical intermediate is a benzyne, formed by elimination of HBr from bromobenzene. Benzyne is too reactive to be isolated as a pure compound but, in the presence of water, addition occurs to give phenol. In the presence of a diene, Diels–Alder cycloaddition takes place.
The electronic structure of benzyne, shown in Figure 16.20, is that of a highly distorted alkyne. Although a typical alkyne triple bond uses sp-hybridized carbon atoms, the benzyne triple bond uses sp2-hybridized carbons. Furthermore, a typical alkyne triple bond has two mutually perpendicular π bonds formed by p–p overlap, but the benzyne triple bond has one π bond formed by p–p overlap and one π bond formed by sp2–sp2 overlap. The latter π bond is in the plane of the ring and is very weak.
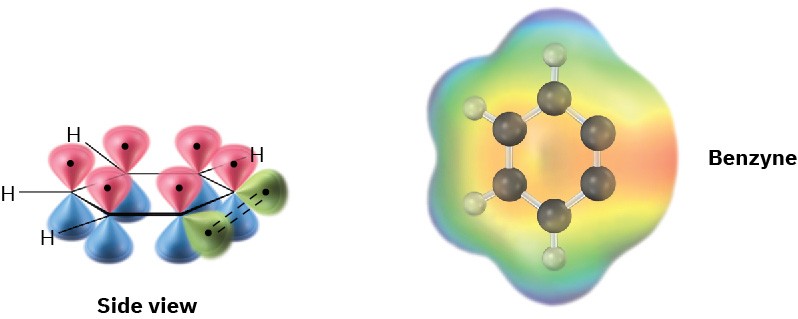
Figure 16.20 An orbital picture and electrostatic potential map of benzyne. The benzyne carbons are sp2-hybridized, and the “third” bond results from weak overlap of two adjacent sp2 orbitals.
Problem 16-17
Treatment of p-bromotoluene with NaOH at 300 °C yields a mixture of two products, but treatment of m-bromotoluene with NaOH yields a mixture of three products. Explain.

