30.2 Electrocyclic Reactions
The best way to understand how orbital symmetry affects pericyclic reactions is to look at some examples. Let’s look first at a group of polyene rearrangements called electrocyclic reactions. An electrocyclic reaction is a pericyclic process that involves the cyclization of a conjugated acyclic polyene. One π bond is broken, the other π bonds change position, a new σ bond is formed, and a cyclic compound results. For example, a conjugated triene can be converted into a cyclohexadiene, and a conjugated diene can be converted into a cyclobutene.
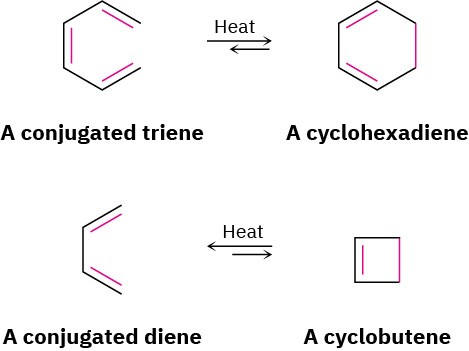
Pericyclic reactions are reversible, and the position of the equilibrium depends on the specific case. In general, the triene ⇄ cyclohexadiene equilibrium favors the cyclic product, whereas the diene ⇄ cyclobutene equilibrium favors the less strained open-chain product.
The most striking feature of electrocyclic reactions is their stereochemistry. For example, (2E,4Z,6E)-2,4,6-octatriene yields only cis-5,6-dimethyl-1,3-cyclohexadiene when heated, and (2E,4Z,6Z)-2,4,6-octatriene yields only trans-5,6-dimethyl-1,3-cyclohexadiene.
Remarkably, however, the stereochemical results change completely when the reactions are carried out under what are called photochemical, rather than thermal, conditions.
Irradiation of (2E,4Z,6E)-2,4,6-octatriene with ultraviolet light (denoted hν) yields trans– 5,6-dimethyl-1,3-cyclohexadiene (Figure 30.4).
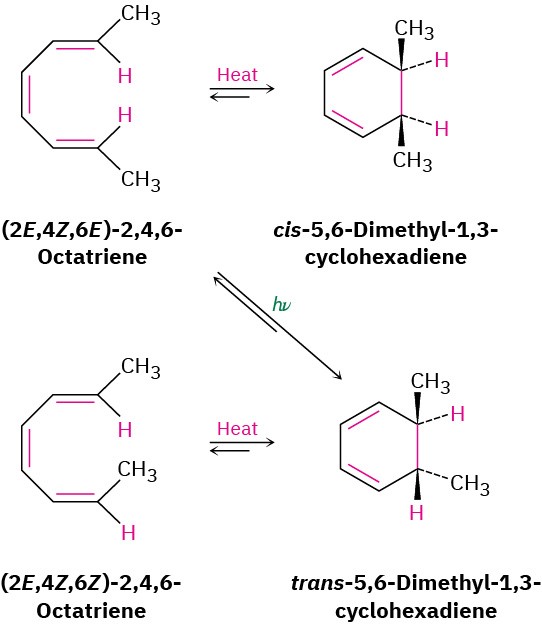
Figure 30.4Electrocyclic interconversions of 2,4,6-octatriene isomers and 5,6- dimethyl-1,3-cyclohexadiene isomers.
A similar result is obtained for the thermal electrocyclic ring-opening of 3,4- dimethylcyclobutene. The trans isomer yields only (2E,4E)-2,4-hexadiene when heated, and the cis isomer yields only (2E,4Z)-2,4-hexadiene. On UV irradiation, however, the results are opposite. Cyclization of the 2E,4E isomer under photochemical conditions yields cis product (Figure 30.5).
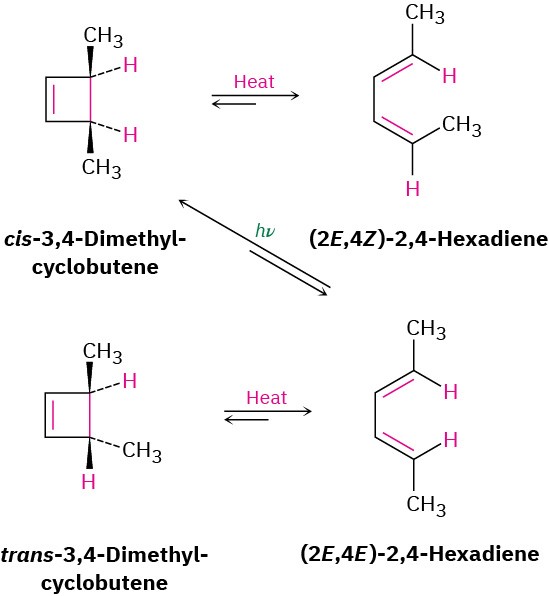
Figure 30.5Electrocyclic interconversions of 2,4-hexadiene isomers and 3,4- dimethylcyclobutene isomers.
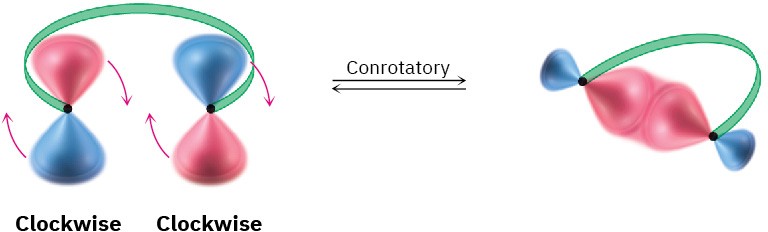
To account for these results, we need to look at the two outermost lobes of the polyene MOs—the lobes that interact when cyclization occurs. There are two possibilities: lobes of like sign can be either on the same side of the molecule or on opposite sides.
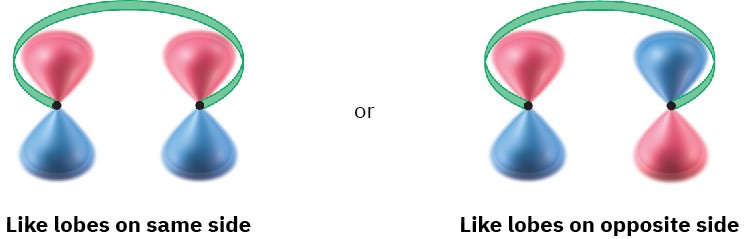
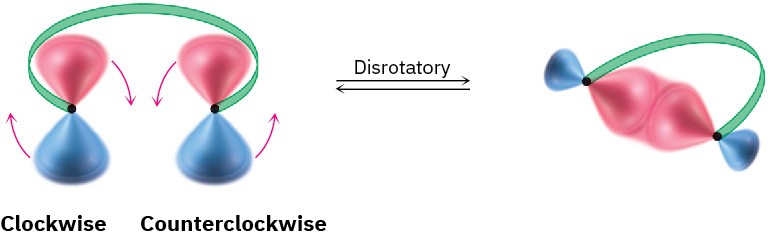
For a bond to form, the outermost π lobes must rotate so that favorable bonding interaction is achieved—a positive lobe with a positive lobe or a negative lobe with a negative lobe. If two lobes of like sign are on the same side of the molecule, the two orbitals must rotate in opposite directions—one clockwise and one counterclockwise. This kind of motion is referred to as disrotatory.
Conversely, if lobes of like sign are on opposite sides of the molecule, both orbitals must rotate in the same direction, either both clockwise or both counterclockwise. This kind of motion is called conrotatory.