16.6 Nucleophilic Aromatic Substitution
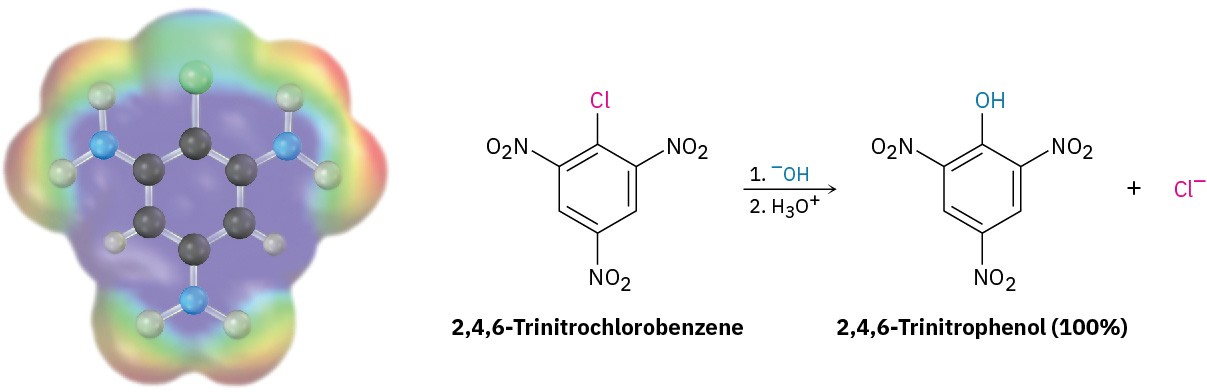
Although aromatic substitution reactions usually occur by an electrophilic mechanism, aryl halides that have electron-withdrawing substituents can also undergo a nucleophilic substitution reaction. For example, 2,4,6-trinitrochlorobenzene reacts with aqueous NaOH at room temperature to give 2,4,6-trinitrophenol. Here, the nucleophile OH– substitutes for Cl–.
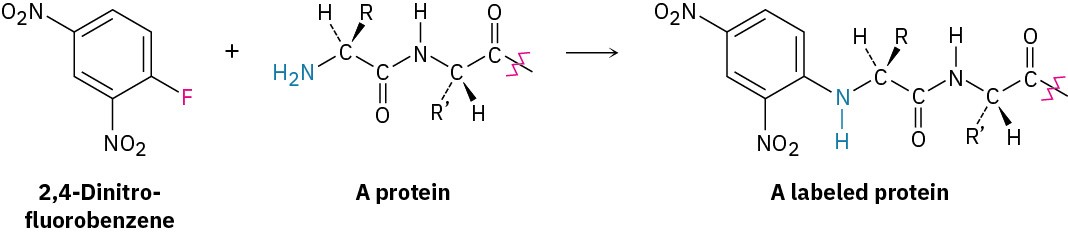
Nucleophilic aromatic substitution is much less common than electrophilic substitution but nevertheless does have certain uses. One such use is the reaction of proteins with 2,4- dinitrofluorobenzene, known as Sanger’s reagent, to attach a “label” to the terminal NH2 group of the amino acid at one end of the protein chain.
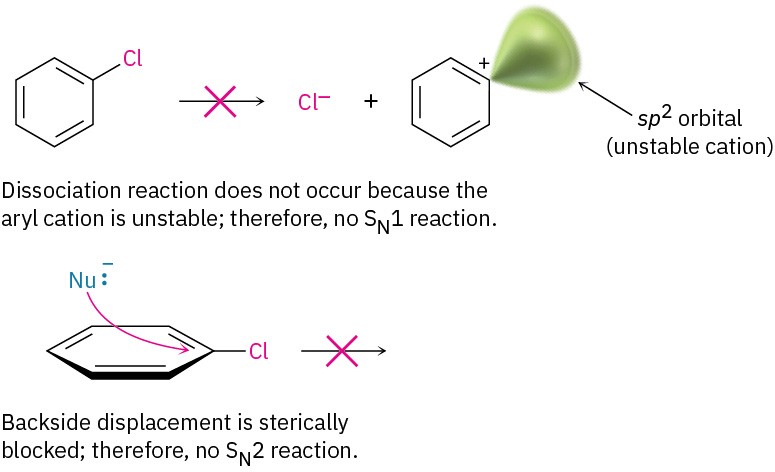
Although the reaction appears superficially similar to the SN1 and SN2 nucleophilic substitutions of alkyl halides discussed in the chapter on Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations, it must be different because aryl halides are inert to both SN1 and SN2 conditions. SN1 reactions don’t occur with aryl halides because dissociation of the halide is energetically unfavorable, due to the instability of the potential aryl cation product. SN2 reactions don’t occur with aryl halides because the halo- substituted carbon of the aromatic ring is sterically shielded from a backside approach. For a nucleophile to react with an aryl halide, it would have to approach directly through the aromatic ring and invert the stereochemistry of the aromatic ring carbon—a geometric impossibility.
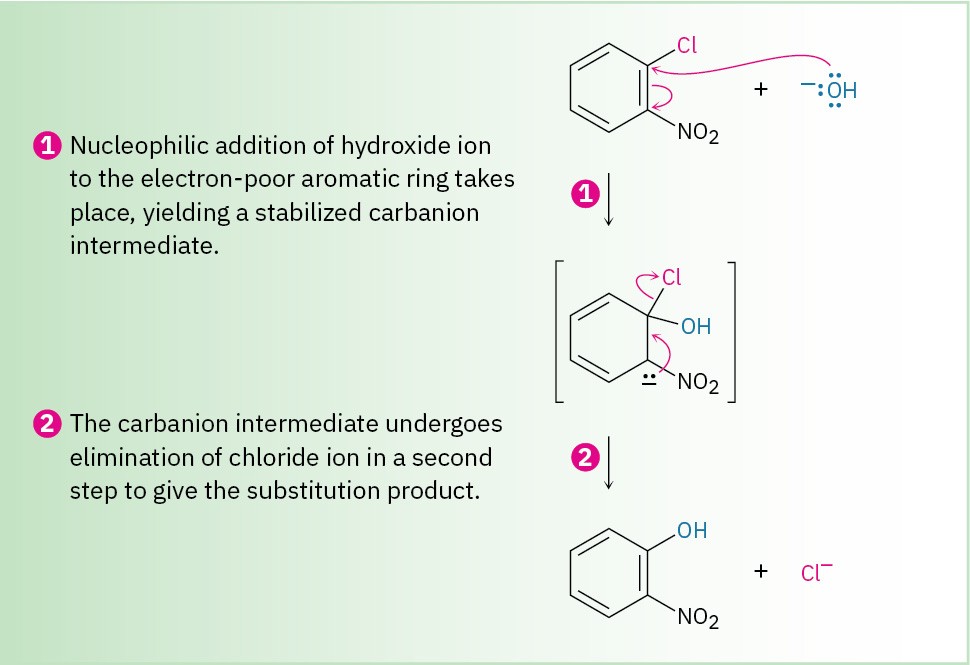
Instead, nucleophilic substitutions on an aromatic ring proceed by the mechanism shown in Figure 16.18. The nucleophile first adds to the electron-deficient aryl halide, forming a resonance-stabilized, negatively charged intermediate called a Meisenheimer complex after its discoverer. Halide ion is then eliminated.
Figure 16.18 MECHANISM
Mechanism of nucleophilic aromatic substitution. The reaction occurs in two steps and involves a resonance-stabilized carbanion intermediate.
Nucleophilic aromatic substitution occurs only if the aromatic ring has an electron- withdrawing substituent in a position ortho or para to the leaving group to stabilize the anion intermediate through resonance (Figure 16.19). A meta substituent offers no such resonance stabilization. Thus, p-chloronitrobenzene and o-chloronitrobenzene react with
hydroxide ion at 130 °C to yield substitution products, but m-chloronitrobenzene is inert to OH–.
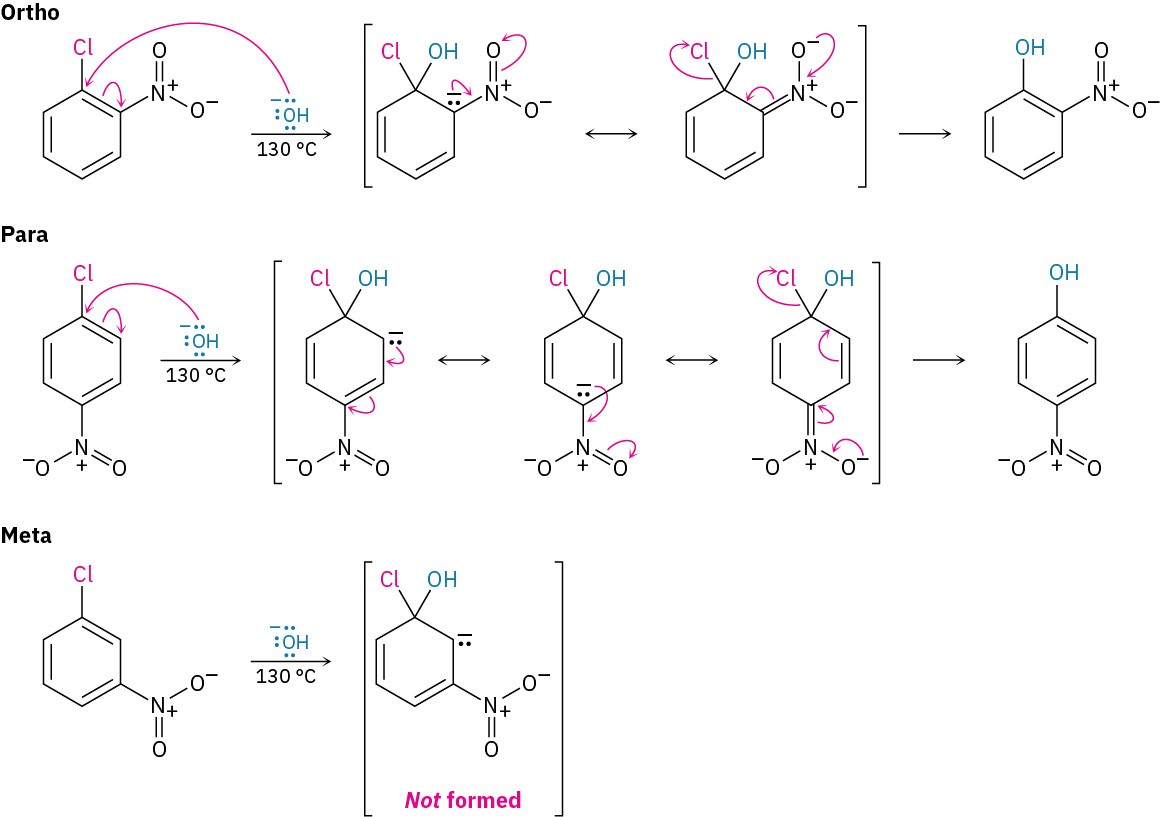
Figure 16.19 Nucleophilic aromatic substitution on nitrochlorobenzenes. Only in the ortho and para intermediates is the negative charge stabilized by a resonance interaction with the nitro group, so only the ortho and para isomers undergo reaction.
Note the differences between electrophilic and nucleophilic aromatic substitutions. Electrophilic substitutions are favored by electron-donating substituents, which stabilize a carbocation intermediate, while nucleophilic substitutions are favored by electron- withdrawing substituents, which stabilize a carbanion intermediate. Thus, the electron- withdrawing groups that deactivate rings for electrophilic substitution (nitro, carbonyl, cyano, and so forth) activate them for nucleophilic substitution. What’s more, these functional groups are meta directors in electrophilic substitution but are ortho–para directors in nucleophilic substitution. And finally, electrophilic substitutions replace hydrogen on the ring, while nucleophilic substitutions replace a leaving group, usually halide ion.
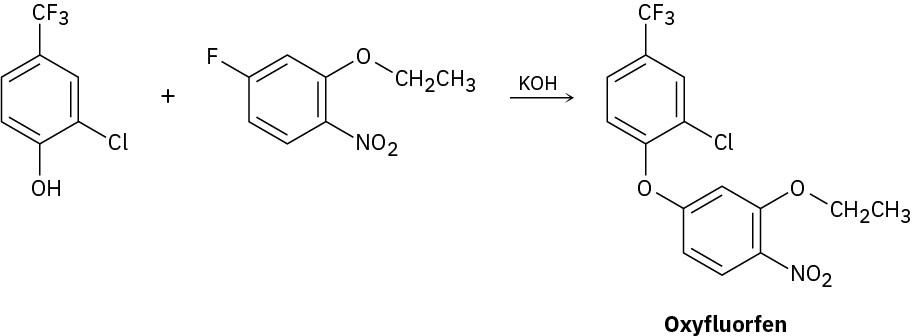
Problem 16-16
The herbicide oxyfluorfen can be prepared by reaction between a phenol and an aryl fluoride. Propose a mechanism.