Chemistry Matters — A Prologue to Metabolism
Biochemistry is carbonyl chemistry. Almost all metabolic pathways used by living organisms involve one or more of the four fundamental carbonyl-group reactions we’ve seen in the chapters on Aldehydes and Ketones: Nucleophilic Addition Reactions through Carbonyl Condensation Reactions. The digestion and metabolic breakdown of all the major classes of food molecules—fats, carbohydrates, and proteins—take place by nucleophilic addition reactions, nucleophilic acyl substitutions, α substitutions, and carbonyl condensations. Similarly, hormones and other crucial biological molecules are built up from smaller precursors by these same carbonyl-group reactions.

Figure 23.10 You are what you eat. Food molecules are metabolized by pathways that involve the four major carbonyl-group reactions. (credit: “Spring” by Giuseppe Arcimboldo, Louvre Museum/Wikimedia Commons, Public Domain)
Take glycolysis, for example, the metabolic pathway by which organisms convert glucose to pyruvate as the first step in extracting energy from carbohydrates.
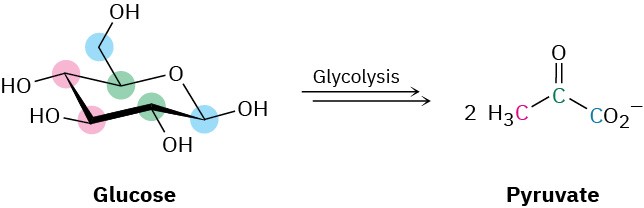
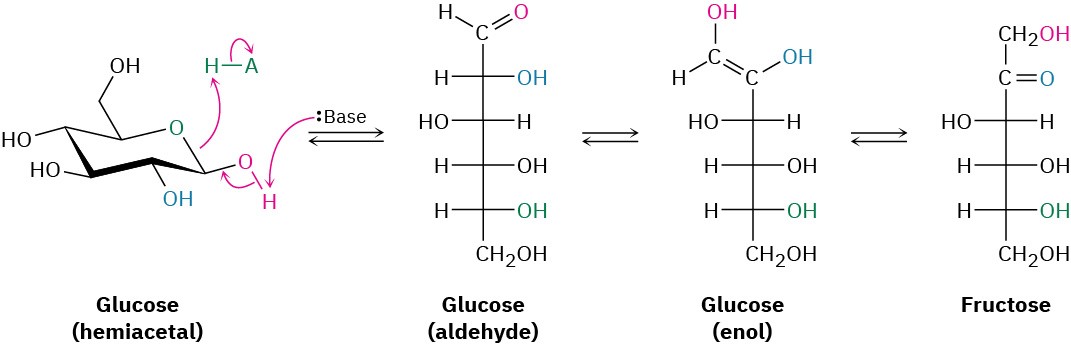
Glycolysis is a ten-step process that begins with isomerization of glucose from its cyclic hemiacetal form to its open-chain aldehyde form—the reverse of a nucleophilic addition reaction. The aldehyde then undergoes tautomerization to yield an enol, which undergoes yet another tautomerization to give the ketone fructose.
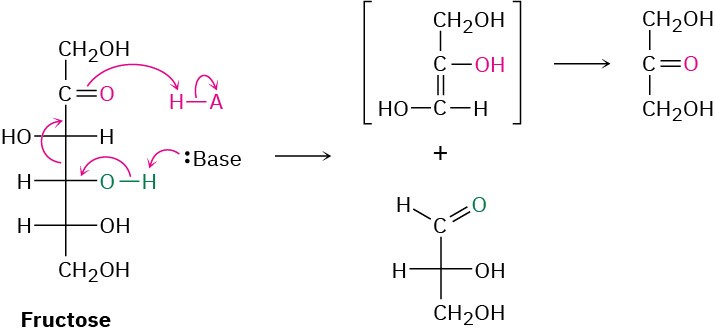
Fructose, a β-hydroxy ketone, is then cleaved by a retro-aldol reaction into two three- carbon molecules—one ketone and one aldehyde. Further carbonyl-group reactions then occur until pyruvate is formed.
These few examples are only an introduction; we’ll look at several of the major metabolic pathways in more detail in Chapter 29. The bottom line, however, is that you haven’t seen the end of carbonyl-group chemistry if you have any interest in biological or medical fields. A solid grasp of carbonyl-group reactions is crucial to an understanding of biochemistry.

