24.2 Structure and Properties of Amines
The bonding in alkylamines is similar to the bonding in ammonia. The nitrogen atom is sp3- hybridized, with its three substituents occupying three corners of a regular tetrahedron and the lone pair of electrons occupying the fourth corner. As you might expect, the C–N–C bond angles are close to the 109° tetrahedral value. For trimethylamine, the C–N–C bond angle is 108° and the C–N bond length is 147 pm.
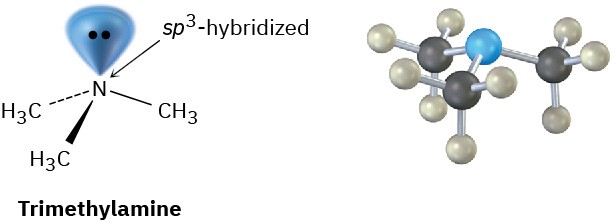
One consequence of tetrahedral geometry is that an amine with three different substituents on nitrogen is chiral, as we saw in Section 5.10. Unlike chiral carbon compounds, however, chiral amines can’t usually be resolved because the two enantiomeric forms rapidly interconvert by a pyramidal inversion, much as an alkyl halide inverts in an SN2 reaction.
Pyramidal inversion occurs by a momentary rehybridization of the nitrogen atom to planar, sp2 geometry, followed by rehybridization of the planar intermediate to tetrahedral, sp3 geometry (Figure 24.2). The barrier to inversion is about 25 kJ/mol (6 kcal/mol), an amount only twice as large as the barrier to rotation about a C–C single bond.
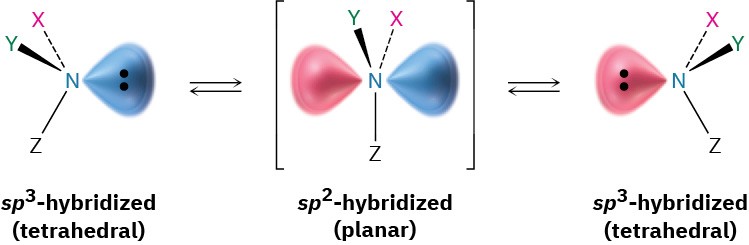
Figure 24.2 Pyramidal inversion rapidly interconverts the two mirror-image (enantiomeric) forms of an amine.
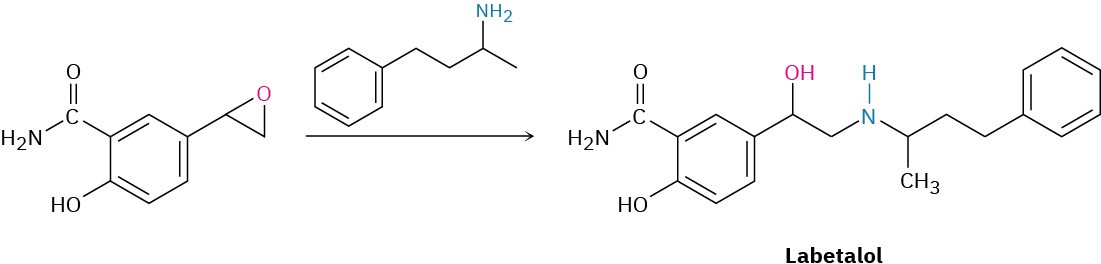
Alkylamines have a variety of applications in the chemical industry as starting materials for the preparation of insecticides and pharmaceuticals. Labetalol, for instance, a so-called β– blocker used for the treatment of high blood pressure, is prepared by SN2 reaction of an epoxide with a primary amine. The substance marketed for drug use is a mixture of all four possible stereoisomers, but the biological activity results primarily from the (R,R) isomer.
Like alcohols, amines with fewer than five carbon atoms are generally water-soluble. Also like alcohols, primary and secondary amines form hydrogen bonds and are highly associated. As a result, amines have higher boiling points than alkanes of similar molecular weight. Diethylamine (MW = 73 amu) boils at 56.3 °C, for instance, while pentane (MW = 72 amu) boils at 36.1 °C.
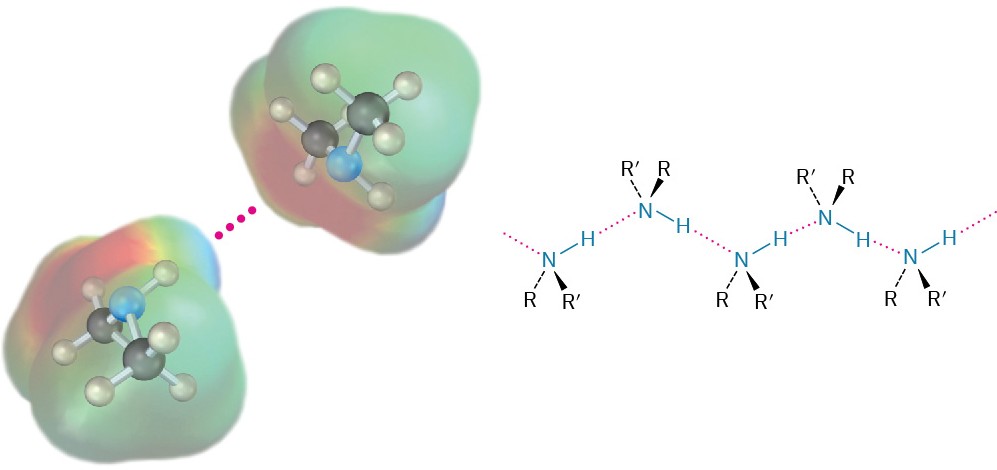
Figure 24.3 Hydrogen bonding in amines.
One other characteristic of amines is their odor. Low-molecular-weight amines such as trimethylamine have a distinctive fishlike aroma, while diamines such as cadaverine (1,5- pentanediamine) and putrescine (1,4-butanediamine) have the appalling odors you might expect from their common names. Both of these diamines arise from the decomposition of decaying tissue.

