21.10 Spectroscopy of Carboxylic Acid Derivatives
Infrared Spectroscopy
All carbonyl-containing compounds have intense IR absorptions in the range 1650 to 1850 cm–1. As shown in Table 21.3 the exact position of the absorption provides information about the specific kind of carbonyl group. For comparison, the IR absorptions of aldehydes, ketones, and carboxylic acids are included in the table, along with values for carboxylic acid derivatives.
Table 21.3 Infrared Absorptions of Some Carbonyl Compounds
|
Example |
Absorption (cm–1) |
|
|
Saturated acid chloride |
Acetyl chloride |
1810 |
|
Aromatic acid chloride |
Benzoyl chloride |
1770 |
|
Saturated acid anhydride |
Acetic anhydride |
1820, 1760 |
|
Saturated ester |
Ethyl acetate |
1735 |
|
Aromatic ester |
Ethyl benzoate |
1720 |
|
Saturated amide |
Acetamide |
1690 |
|
Aromatic amide |
Benzamide |
1675 |
|
N-Substituted amide |
N-Methylacetamide |
1680 |
|
N,N-Disubstituted amide |
N,N-Dimethylacetamide |
1650 |
|
Saturated aldehyde |
Acetaldehyde |
1730 |
|
Saturated ketone |
Acetone |
1715 |
|
Saturated carboxylic acid |
Acetic acid |
1710 |
Acid chlorides are easily detected by their characteristic absorption near 1810 cm–1. Acid anhydrides can be identified by a pair of absorptions in the carbonyl region, one at 1820 cm–1 and another at 1760 cm–1. Note that each of these functional groups has a strong electron-withdrawing group attached to the carbonyl. The inductive withdrawal of electron density shortens the C=O bond, thereby raising its stretching frequency. Esters are detected by their absorption at 1735 cm–1, a position somewhat higher than that for either aldehydes or ketones. Amides, by contrast, absorb near the low-wavenumber end of the carbonyl region, with the degree of substitution on nitrogen affecting the exact position of the IR band. That is, for amides, the delocalization of electron density (resonance) from nitrogen into the carbonyl lengthens the C=O bond and lowers its stretching frequency.
Problem 21-25
What kinds of functional groups might compounds have if they show the following IR absorptions?
(a)
Absorption at 1735 cm–1 (b)
Absorption at 1810 cm–1 (c)
Absorptions at 2500 to 3300 cm–1 and 1710 cm–1 (d)
Absorption at 1715 cm–1 Problem 21-26
Propose structures for compounds that have the following formulas and IR absorptions: (a)
C6H12O2, 1735 cm–1
(b)
C4H9NO, 1650 cm–1
(c)
C4H5ClO, 1780 cm–1
Nuclear Magnetic Resonance Spectroscopy
Hydrogens on the carbon next to a carbonyl group are slightly deshielded and absorb near 2 δ in the 1H NMR spectrum, although the exact identity of the carbonyl group can’t be determined. Figure 21.11 shows the 1H NMR spectrum of ethyl acetate.
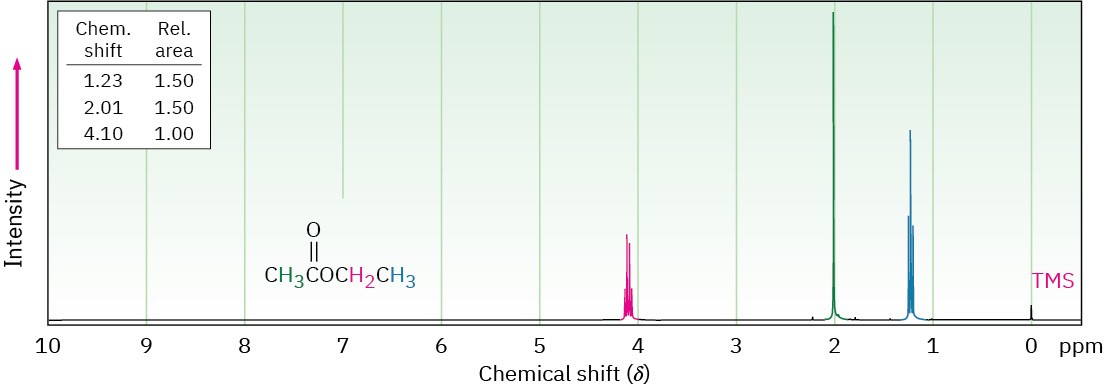
Figure 21.111H NMR spectrum of ethyl acetate.
Although 13C NMR is useful for determining the presence or absence of a carbonyl group in a molecule, the identity of the carbonyl group is difficult to determine. Aldehydes and ketones absorb near 200 δ, while the carbonyl carbon atoms of various acid derivatives absorb in the range 160 to 180 δ (Table 21.4).
Table 21.4 13C NMR Absorptions of Some Carbonyl Compounds
|
Absorption (δ) |
|
|
Acetic acid |
177.3 |
|
Ethyl acetate |
170.7 |
|
Acetyl chloride |
170.3 |
|
Acetamide |
172.6 |
|
Acetic anhydride |
166.9 |
|
Acetone |
205.6 |
|
Acetaldehyde |
201.0 |
Additional Problems 21 • Additional Problems 21 • Additional Problems Visualizing Chemistry Problem 21-27
Name the following compounds:
(a)
(b)
Problem 21-28
How would you prepare the following compounds starting with an appropriate carboxylic acid and any other reagents needed? (reddish brown = Br.)
(a)
(b)
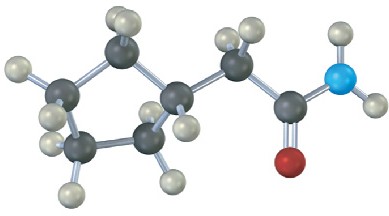
Problem 21-29
The following structure represents a tetrahedral alkoxide-ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting acid derivative, and the ultimate product (green = Cl).
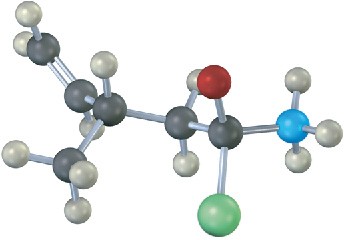
Problem 21-30
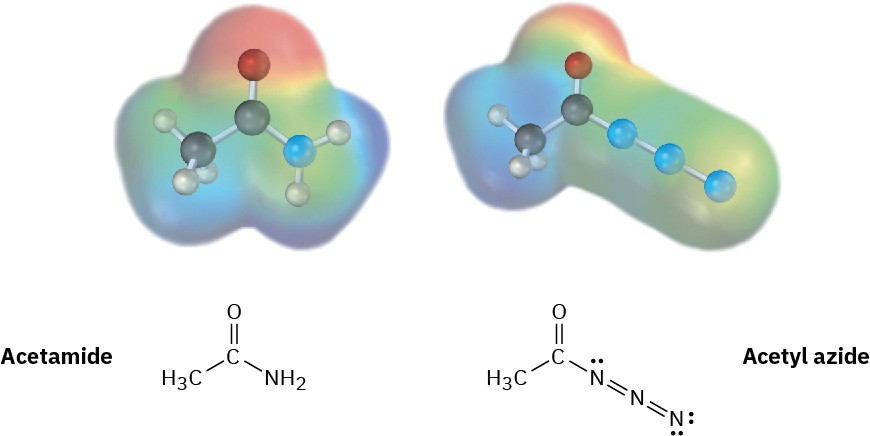
Electrostatic potential maps of a typical amide (acetamide) and an acyl azide (acetyl azide) are shown. Which of the two do you think is more reactive in nucleophilic acyl substitution reactions? Explain.
Mechanism Problems
Problem 21-31
Predict the product(s) and write the mechanism for the following reactions: (a)
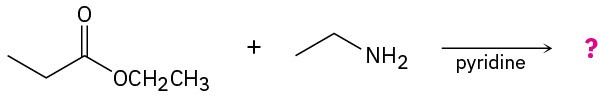
(b)
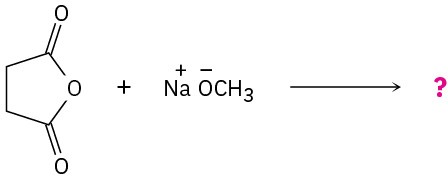
Problem 21-32
Predict the product(s) and write the mechanism for the following reactions: (a)
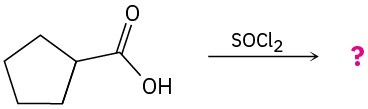
(b)
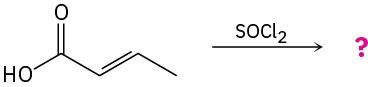
Problem 21-33
Predict the product(s) and write the mechanism for each of the following reactions: (a)
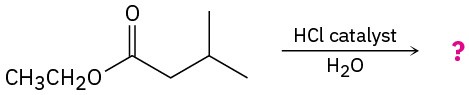
(b)
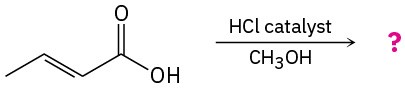
Problem 21-34
Predict the product(s) and write the mechanism for the following reactions: (a)
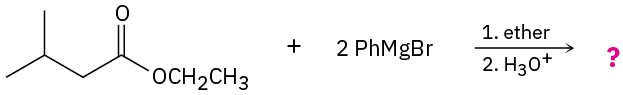
(b)
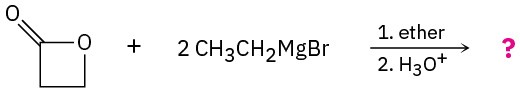
Problem 21-35
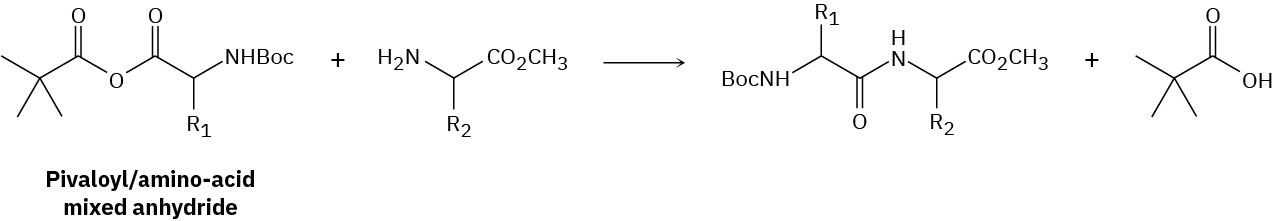
Pivalic mixed anhydrides are often used to form amide bonds between amino acids. Unlike with a symmetrical anhydride, this reaction is highly regioselective, with the nucleophile adding only to the amino-acid carbonyl. Provide the complete mechanism for the following reaction and explain the regioselectivity.
Problem 21-36
When 4-dimethylaminopyridine (DMAP) is added in catalytic amounts to acetic anhydride and an alcohol, it significantly increases the rate of ester formation. The process begins with a reaction between acetic anhydride and DMAP to form a highly reactive acetylpyridinium intermediate that is more reactive than acetic anhydride itself. Propose a mechanism for this process that includes the formation and reaction of the acetylpyridinium intermediate.
Problem 21-37
Fats are biosynthesized from glycerol 3-phosphate and fatty-acyl CoA’s by a reaction sequence that begins with the following step. Show the mechanism of the reaction.
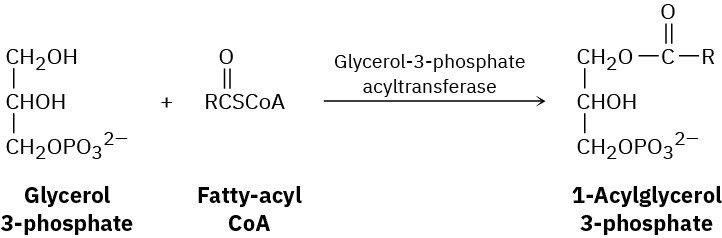
Problem 21-38
Treatment of an α-amino acid with DCC yields a 2,5-diketopiperazine. Propose a mechanism.
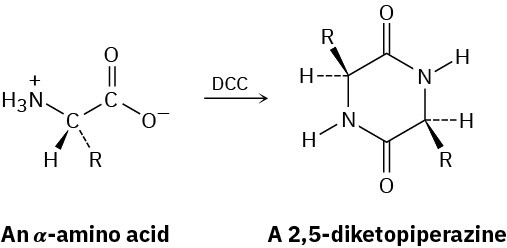
Problem 21-39
Succinic anhydride yields the cyclic imide succinimide when heated with ammonium chloride at 200 °C. Propose a mechanism for this reaction. Why do you suppose such a high reaction temperature is required?
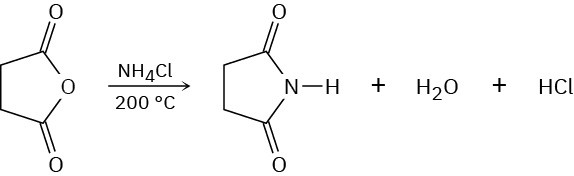
Problem 21-40
The hydrolysis of a biological thioester to the corresponding carboxylate is often more complex than the overall result might suggest. The conversion of succinyl CoA to succinate in the citric acid cycle, for instance, occurs by initial formation of an acyl phosphate, followed by reaction with guanosine diphosphate (GDP, a relative of adenosine diphosphate [ADP]) to give succinate and guanosine triphosphate (GTP, a relative of ATP). Suggest mechanisms for both steps.
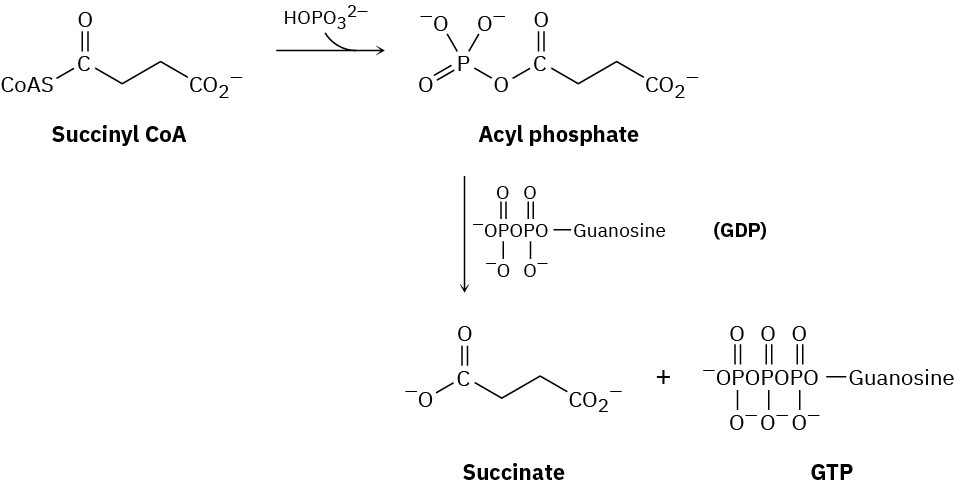
Problem 21-41
One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehyde 3-phosphate. The process occurs by phosphorylation with ATP to give 1,3-bisphosphoglycerate, reaction with a thiol group on the enzyme to give an enzyme-bound thioester, and reduction with NADH. Suggest mechanisms for all three reactions.
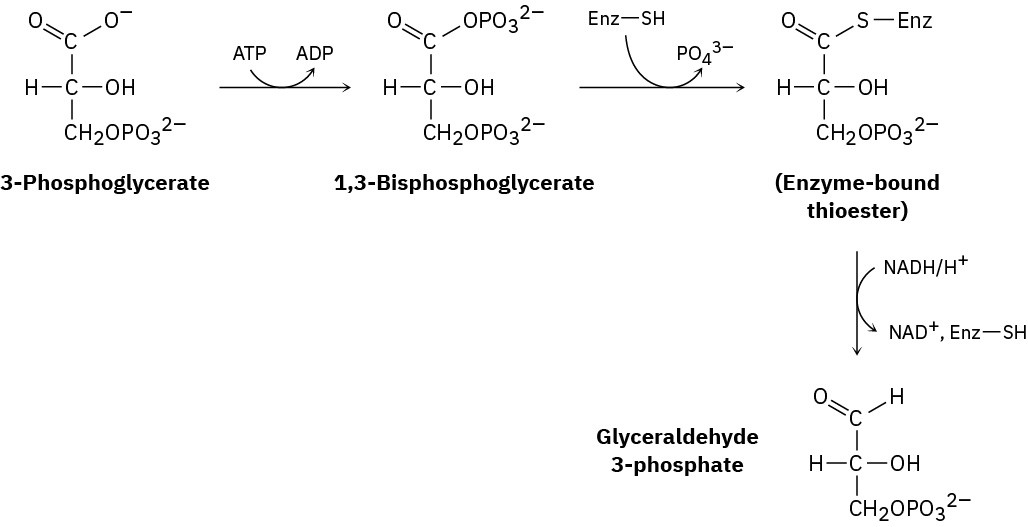
Problem 21-42
Bacteria typically develop a resistance to penicillins and other β-lactam antibiotics (see Chemistry Matters at the end of this chapter) due to bacterial synthesis of β-lactamase enzymes. Tazobactam, however, is able to inhibit the activity of the β-lactamase by trapping it, thereby preventing a resistance from developing.
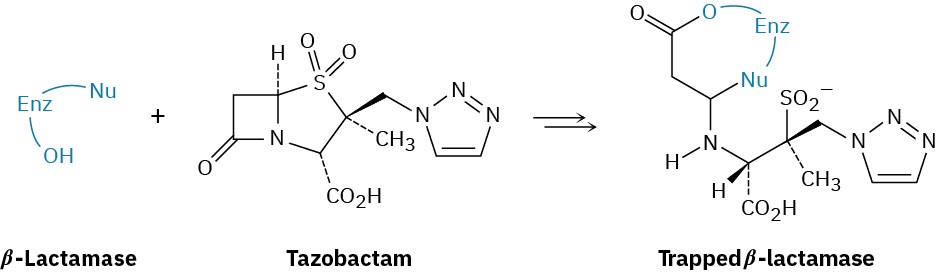
(a)
The first step in trapping is reaction of a hydroxyl group on the β-lactamase to open the β– lactam ring of tazobactam. Show the mechanism.
(b)
The second step is opening the sulfur-containing ring in tazobactam to give an acyclic imine intermediate. Show the mechanism.
(c)
Cyclization of the imine intermediate gives the trapped β-lactamase product. Show the mechanism.
Problem 21-43
The following reaction, called the benzilic acid rearrangement, takes place by typical carbonyl-group reactions. Propose a mechanism (Ph = phenyl).
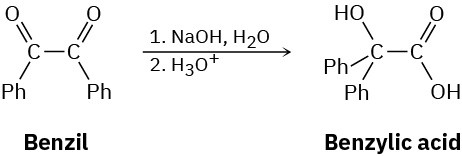
Problem 21-44
In the iodoform reaction, a triiodomethyl ketone reacts with aqueous NaOH to yield a carboxylate ion and iodoform (triiodomethane). Propose a mechanism for this reaction.
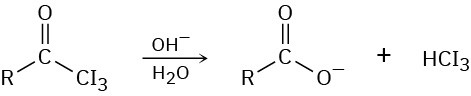
Naming Carboxylic Acid Derivatives
Problem 21-45
Give IUPAC names for the following compounds: (a)
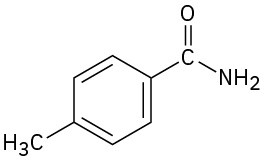
(b)
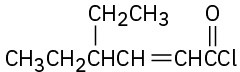
(c)
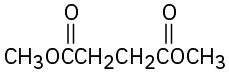
(d)
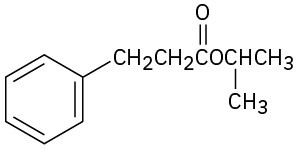
(e)
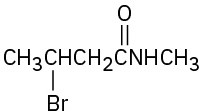
(f)
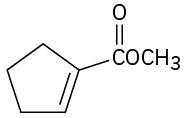
(g)
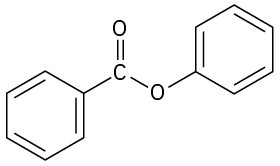
(h)
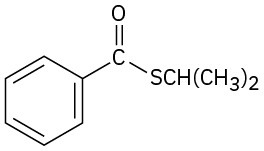
Problem 21-46
Draw structures corresponding to the following names: (a)
p-Bromophenylacetamide (b)
m-Benzoylbenzamide (c)
2,2-Dimethylhexanamide (d)
Cyclohexyl cyclohexanecarboxylate (e)
Ethyl 2-cyclobutenecarboxylate (f)
Succinic anhydride Problem 21-47
Draw and name compounds that meet the following descriptions: (a)
Three acid chlorides having the formula C6H9ClO
(b)
Three amides having the formula C7H11NO Nucleophilic Acyl Substitution Reactions Problem 21-48
Predict the product, if any, of reaction between propanoyl chloride and the following reagents:
(a)
Li(Ph)2Cu in ether (b)
LiAlH4, then H3O+ (c)
CH3MgBr, then H3O+ (d)
H3O+
(e) Cyclohexanol (f)
Aniline (g)
CH3CO2– Na+
Problem 21-49
Answer Problem 21-48 for reaction of the listed reagents with methyl propanoate. Problem 21-50
Answer Problem 21-48 for reaction of the listed reagents with propanamide. Problem 21-51
What product would you expect to obtain from Grignard reaction of an excess of phenylmagnesium bromide with dimethyl carbonate, CH3OCO2CH3?
Problem 21-52
How might you prepare the following compounds from butanoic acid?
(a)
1-Butanol (b) Butanal (c)
1-Bromobutane (d) Pentanenitrile (e)
- Butene (f)
N-Methylpentanamide (g)
- Hexanone (h) Butylbenzene (i) Butanenitrile Problem 21-53
Predict the product(s) of the following reactions: (a)
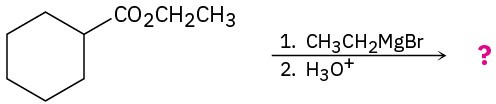
(b)
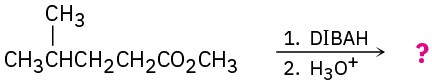
(c)
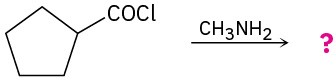
(d)
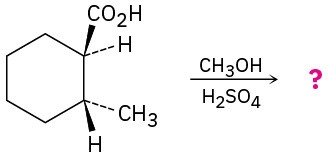
(e)
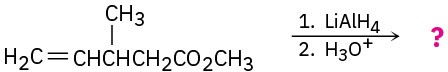
(f)
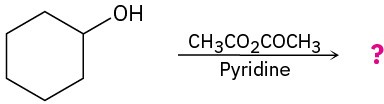
(g)
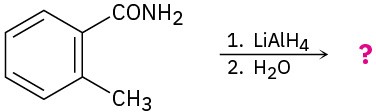
(h)
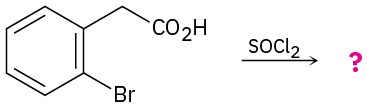
Problem 21-54
The following reactivity order has been found for the saponification of alkyl acetates by aqueous NaOH. Explain.
CH3CO2CH3 > CH3CO2CH2CH3 > CH3CO2CH(CH3)2 > CH3CO2C(CH3)3
Problem 21-55
Explain the observation that attempted Fischer esterification of 2,4,6-trimethylbenzoic acid with methanol and HCl is unsuccessful. No ester is obtained, and the acid is recovered unchanged. What alternative method of esterification might be successful?
Problem 21-56
Outline methods for the preparation of acetophenone (phenyl methyl ketone) starting from the following:
(a) Benzene (b)
Bromobenzene (c)
Methyl benzoate (d)
Benzonitrile (e)
Styrene Problem 21-57
Treatment of 5-aminopentanoic acid with DCC (dicyclohexylcarbodiimide) yields a lactam. Show the structure of the product and the mechanism of the reaction.
Problem 21-58
When ethyl benzoate is heated in methanol containing a small amount of HCl, methyl
benzoate is formed. Propose a mechanism for the reaction.
Problem 21-59
tert-Butoxycarbonyl azide, a reagent used in protein synthesis, is prepared by treating tert– butoxycarbonyl chloride with sodium azide. Propose a mechanism for this reaction.
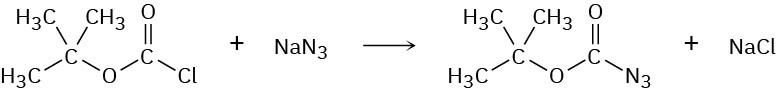
Step-Growth Polymers
Problem 21-60
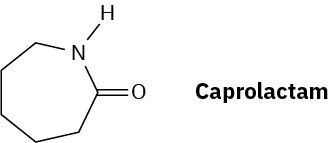
The step-growth polymer nylon 6 is prepared from caprolactam. The reaction involves initial reaction of caprolactam with water to give an intermediate open-chain amino acid, followed by heating to form the polymer. Propose mechanisms for both steps, and show the structure of nylon 6.
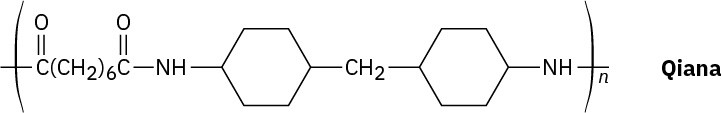
Problem 21-61
Qiana, a polyamide fiber with a silky texture, has the following structure. What are the monomer units used in the synthesis of Qiana?
Problem 21-62
What is the structure of the polymer produced by treatment of β-propiolactone with a small amount of hydroxide ion?
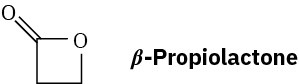
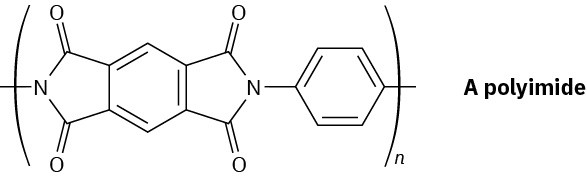
Problem 21-63
Polyimides with the structure shown are used as coatings on glass and plastics to improve scratch resistance. How would you synthesize a polyimide? (See Problem 21-39.)
Spectroscopy
Problem 21-64
How would you distinguish spectroscopically between the following isomer pairs? Tell what differences you would expect to see.
(a)
N-Methylpropanamide and N,N-dimethylacetamide (b)
5-Hydroxypentanenitrile and cyclobutanecarboxamide (c)
4-Chlorobutanoic acid and 3-methoxypropanoyl chloride (d)
Ethyl propanoate and propyl acetate Problem 21-65
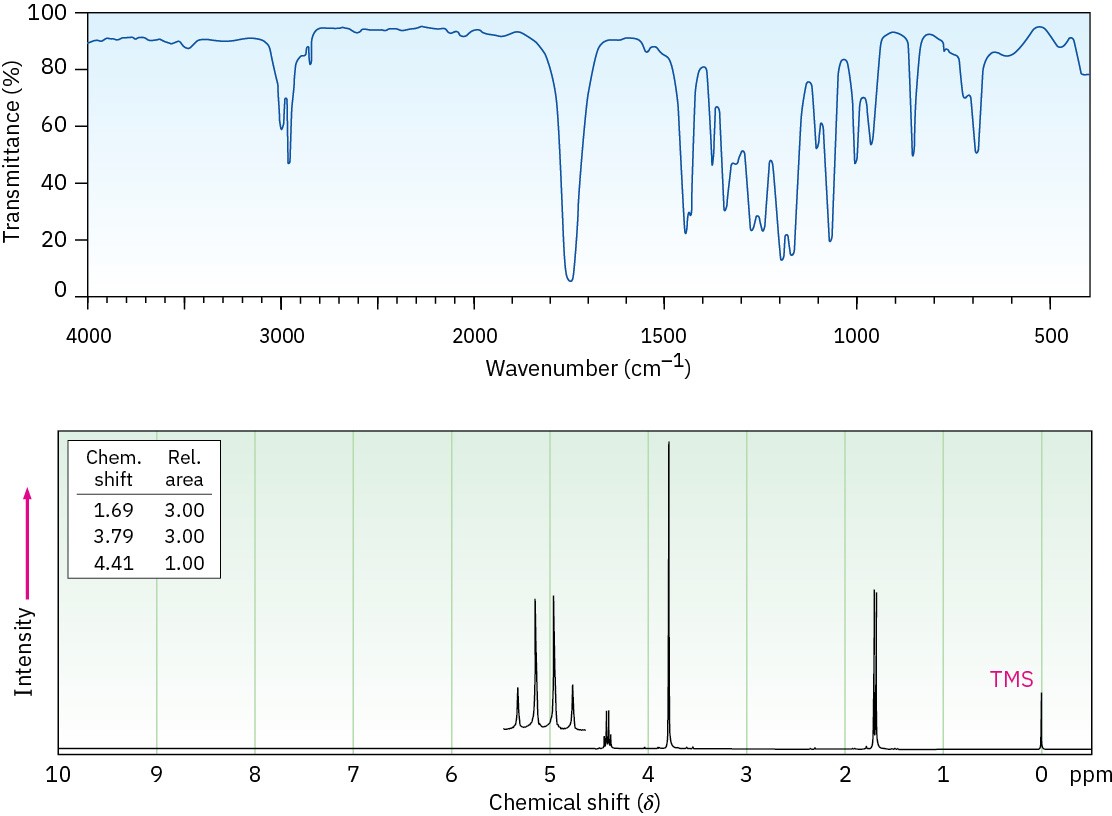
Propose a structure for a compound, C4H7ClO2, that has the following IR and 1H NMR spectra:
Problem 21-66
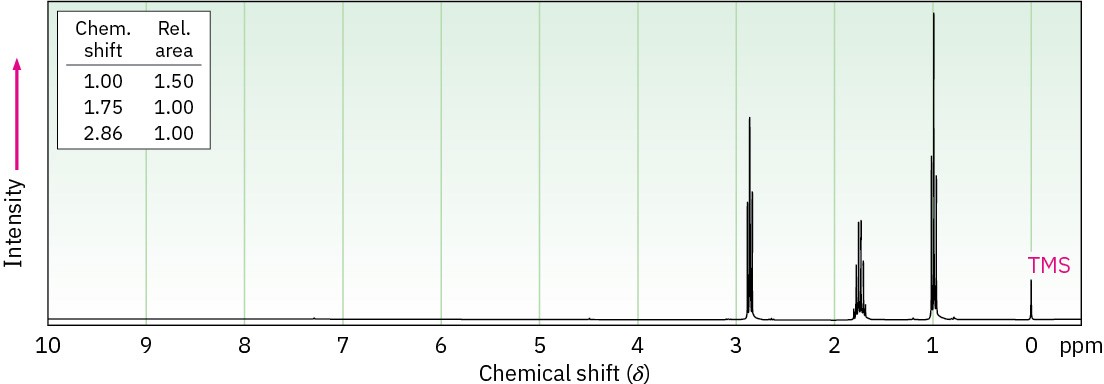
Assign structures to compounds with the following 1H NMR spectra: (a)
C4H7ClO
IR: 1810 cm–1
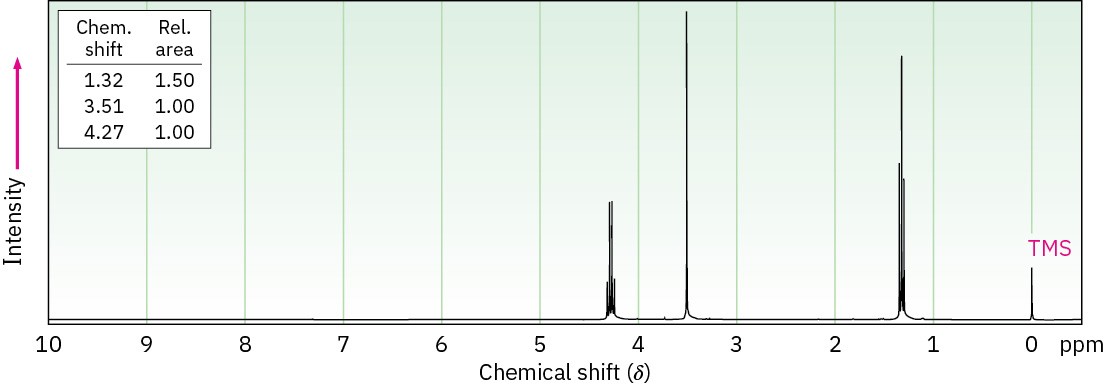
(b) C5H7NO2
IR: 2250, 1735 cm–1
General Problems
Problem 21-67
The following reactivity order has been found for the basic hydrolysis of p-substituted methyl benzoates:
Y=NO2 > Br > H > CH3 > OCH3
How can you explain this reactivity order? Where would you expect Y=C≡N, Y=CHO, and Y=NH2 to be in the reactivity list?
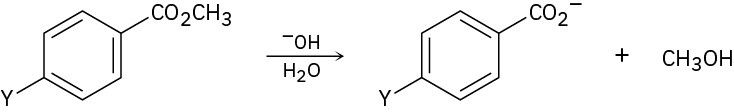
Problem 21-68
When a carboxylic acid is dissolved in isotopically labeled water, the label rapidly becomes incorporated into both oxygen atoms of the carboxylic acid. Explain.
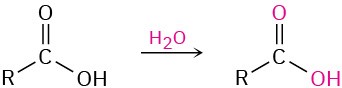
Problem 21-69
We said in Section 21.6 that mechanistic studies on ester hydrolysis have been carried out using ethyl propanoate labeled with 18O in the ether-like oxygen. Assume that 18O-labeled acetic acid is your only source of isotopic oxygen, and then propose a synthesis of the labeled ethyl propanoate.
Problem 21-70
Treatment of a carboxylic acid with trifluoroacetic anhydride leads to an unsymmetrical anhydride that rapidly reacts with alcohol to give an ester.
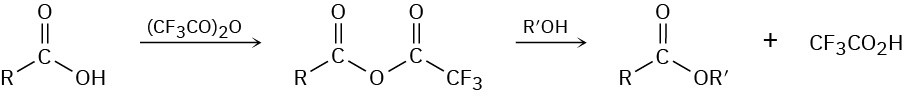
(a)
Propose a mechanism for formation of the unsymmetrical anhydride. (b)
Why is the unsymmetrical anhydride unusually reactive? (c)
Why does the unsymmetrical anhydride react as indicated rather than giving a trifluoroacetate ester plus carboxylic acid?
Problem 21-71
Butacetin is an analgesic (pain-killing) agent that is synthesized commercially from p– fluoronitrobenzene. Propose a synthesis.
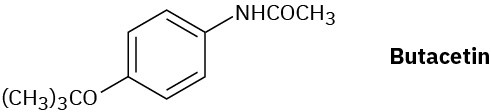
Problem 21-72
Phenyl 4-aminosalicylate is a drug used in the treatment of tuberculosis. Propose a synthesis of this compound starting from 4-nitrosalicylic acid.
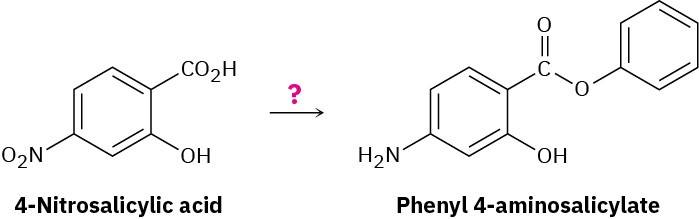
Problem 21-73
N,N-Diethyl-m-toluamide (DEET) is the active ingredient in many insect-repellent preparations. How might you synthesize this substance from m-bromotoluene?
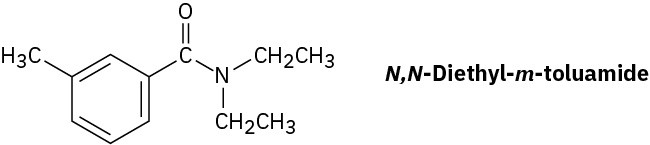
Problem 21-74
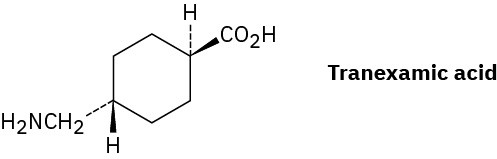
Tranexamic acid, a drug useful against blood clotting, is prepared commercially from p– methylbenzonitrile. Formulate the steps likely to be used in the synthesis. (Don’t worry about cis–trans isomers; heating to 300 °C interconverts the isomers.)
Problem 21-75
One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2.
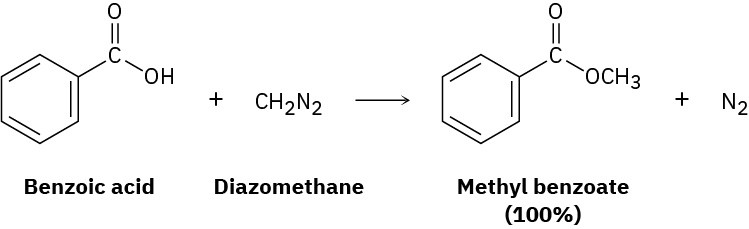
The reaction occurs in two steps: (1) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+.
(a)
Draw two resonance structures of diazomethane, and account for step 1. (b)
What kind of reaction occurs in step 2? Problem 21-76
Draw the structure of the polymer you would expect to obtain from reaction of dimethyl terephthalate with a triol such as glycerol. What structural feature would this new polymer have that was not present in Dacron (Table 21.2)? How do you think this new feature might affect the properties of the polymer?
Problem 21-77
Assign structures to compounds with the following 1H NMR spectra: (a)
C5H10O2
IR: 1735 cm–1
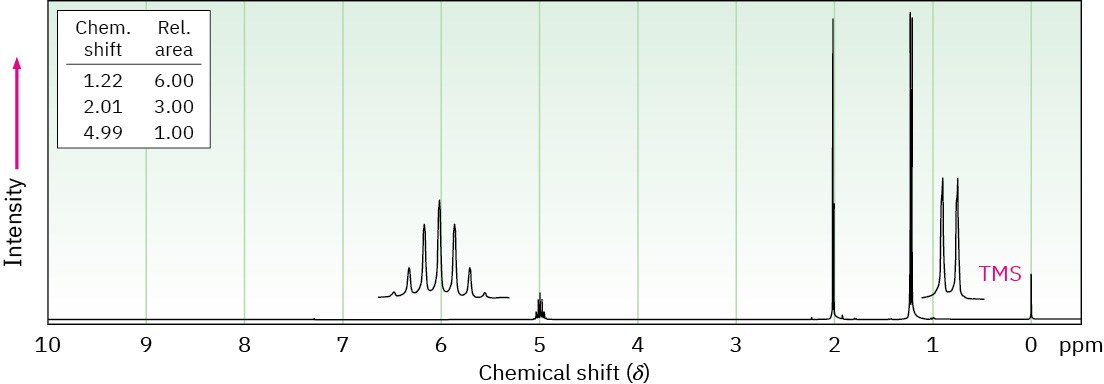
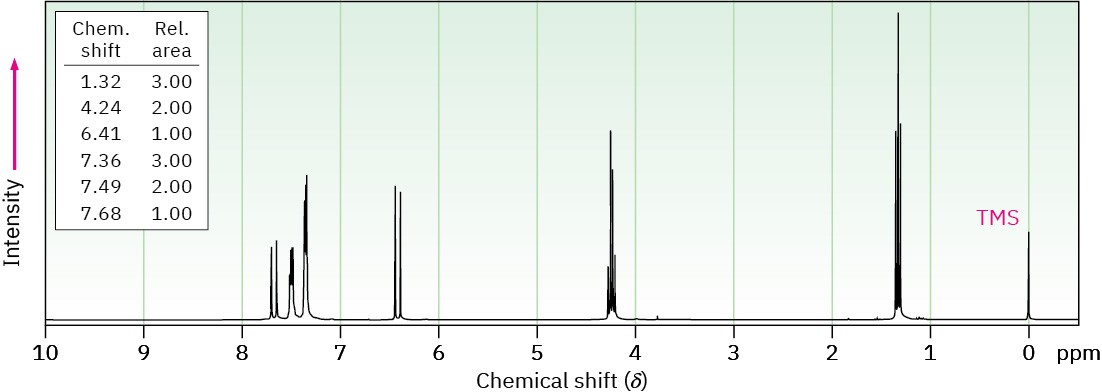
(b) C11H12O2
IR: 1710 cm–1
Problem 21-78
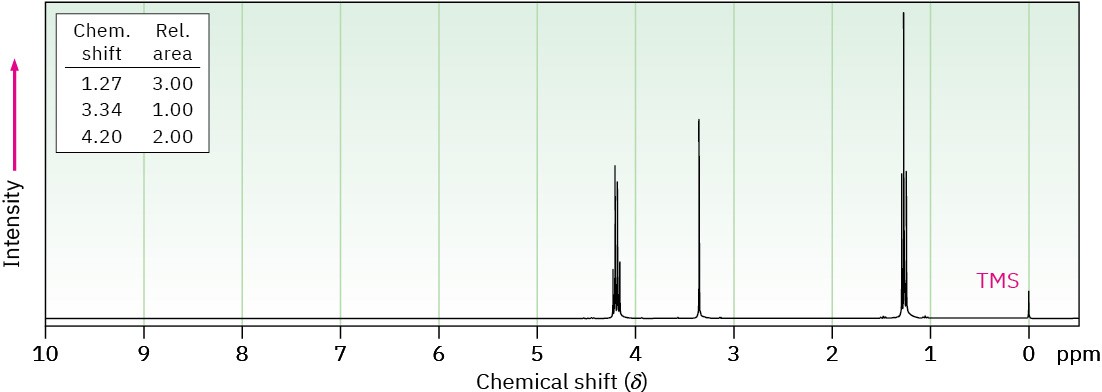
Propose structures for compounds with the following 1H NMR spectra: (a)
C5H9ClO2
IR: 1735 cm–1
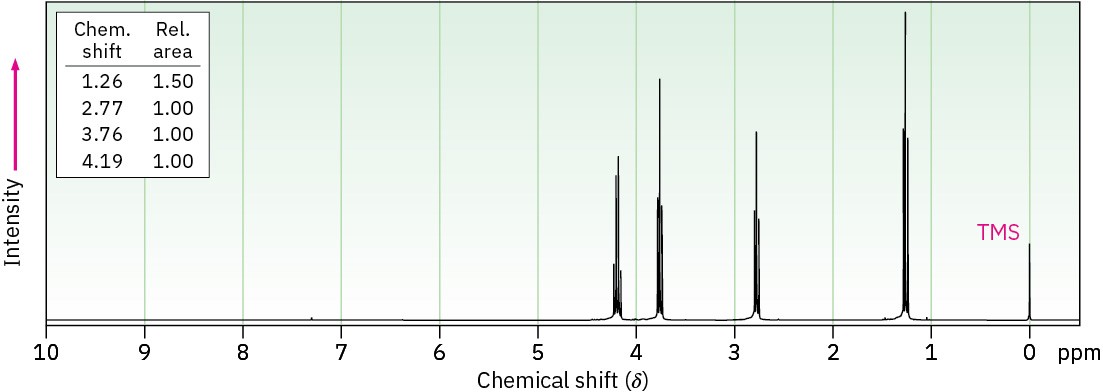
(b) C7H12O4
IR: 1735 cm–1
Problem 21-79
Propose a structure for the compound with the formula C19H9NO2 and the following IR and NMR spectra
Problem 21-80
Draw the structure of the compound that produced the following spectra. The infrared spectrum has strong bands at 1720 and 1738 cm–1.
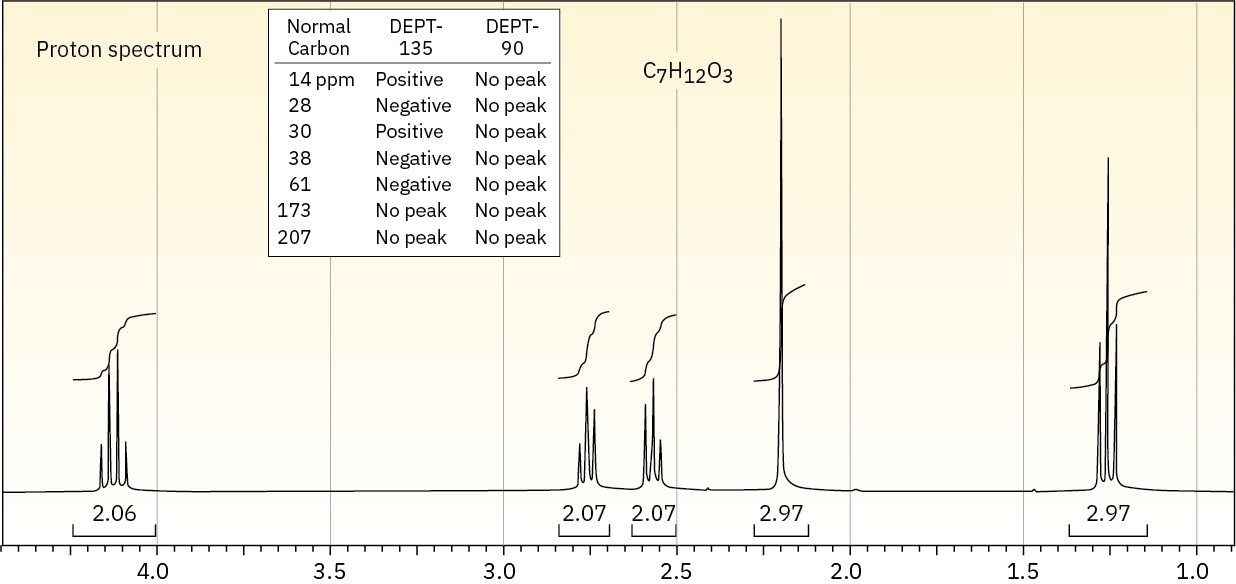
Problem 21-81
When an amide is formed from an acid chloride or an anhydride, two equivalents of base are required. However, when an ester is used as the starting material, only one equivalent of base is needed. Explain this reactivity in terms of basicity of the leaving groups.
Problem 21-82
Epoxy adhesives are prepared in two steps. SN2 reaction of the disodium salt of bisphenol A with epichlorohydrin forms a “prepolymer,” which is then “cured” by treatment with a triamine such as H2NCH2CH2NHCH2CH2NH2.
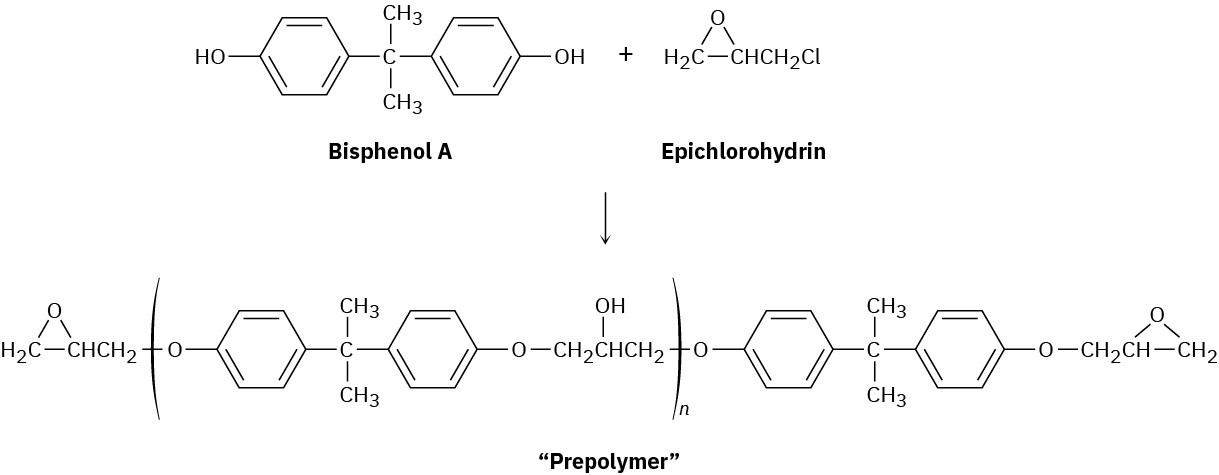
Draw structures to show how addition of the triamine results in a strengthening of the polymer.

