31.4 Step-Growth Polymers
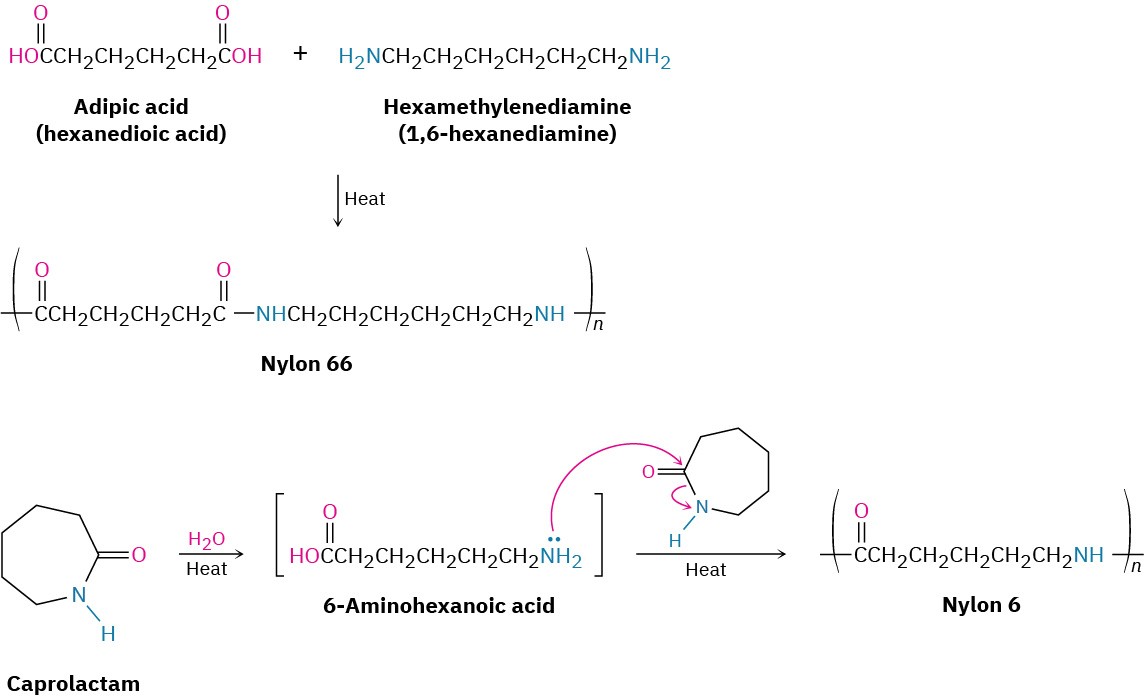
Step-growth polymers are produced by reactions in which each bond in the polymer is formed stepwise, independently of the other bonds. Like the polyamides (nylons) and polyesters that we saw in Section 21.9, most step-growth polymers are produced by reaction between two difunctional reactants. The polymer Nylon 66, for instance, is made by reaction between the six-carbon adipic acid and the six-carbon hexamethylenediamine (1,6-hexanediamine). Alternatively, a single reactant with two different functional groups can polymerize. Nylon 6 is made by polymerization of the six-carbon caprolactam. The reaction is initiated by adding a small amount of water, which hydrolyzes some caprolactam to 6-aminohexanoic acid. Nucleophilic addition of the amino group to caprolactam then propagates the polymerization.
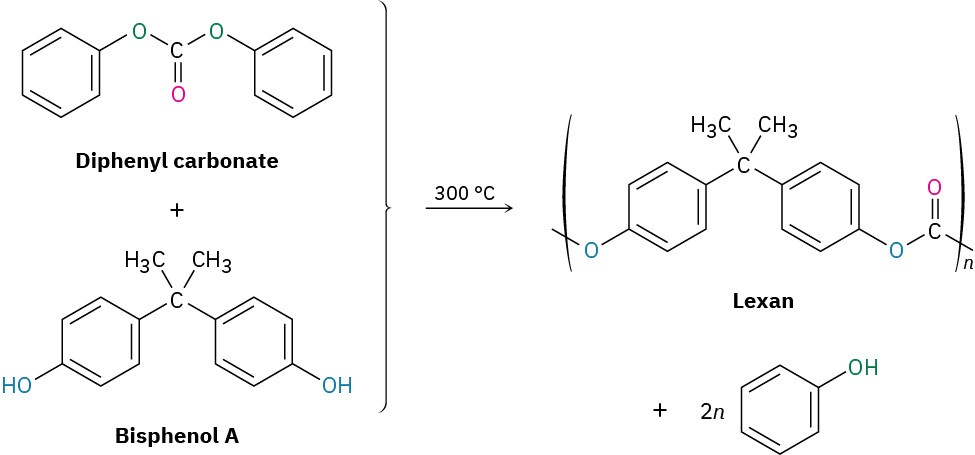
Polycarbonates
Polycarbonates are like polyesters, but their carbonyl group is linked to two –OR groups, [O═C(OR)!]. Lexan, for instance, is a polycarbonate prepared from diphenyl carbonate and a diphenol called bisphenol A. Lexan has unusually high impact strength, making it valuable for use in machinery housings, telephones, bicycle safety helmets, and bulletproof glass.
Polyurethanes
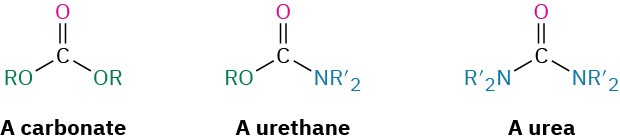
A urethane is a carbonyl-containing functional group in which the carbonyl carbon is bonded to both an –OR group and an –NR2 group. As such, a urethane is halfway between a carbonate and a urea.
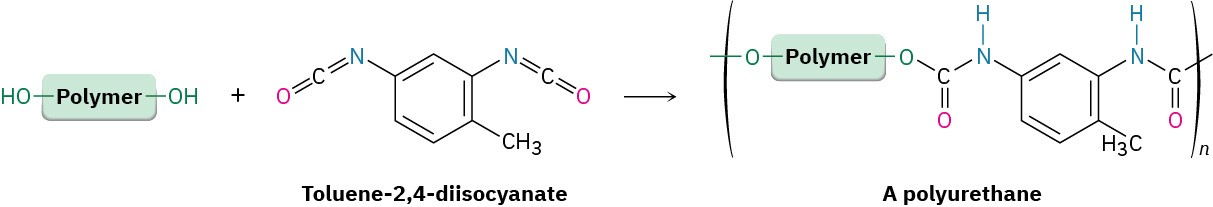
A urethane is typically prepared by nucleophilic addition reaction of an alcohol with an isocyanate (R─N═C═O), so a polyurethane is prepared by reaction of a diol with a diisocyanate. The diol is usually a low-molecular-weight polymer (MW ≈ 1000 amu) with hydroxyl end-groups; the diisocyanate is often toluene-2,4-diisocyanate.
Several different kinds of polyurethanes can be produced, depending on the nature of the polymeric alcohol used. One major use of polyurethane is in the stretchable spandex fibers used for bathing suits and athletic gear. These polyurethanes have a fairly low degree of cross-linking so that the resultant polymer is soft and elastic. A second major use of polyurethanes is in the foams used for insulation. Foaming occurs when a small amount of water is added during polymerization, giving a carbamic acid intermediate that spontaneously loses bubbles of CO2.
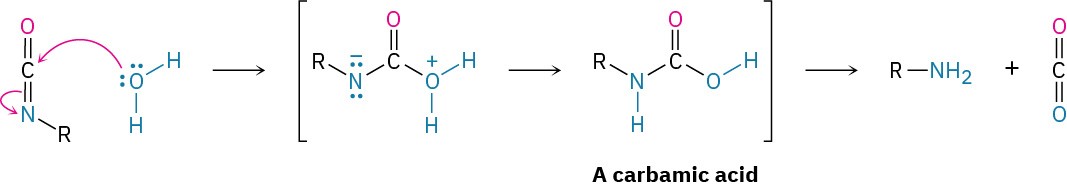
Polyurethane foams are generally made using a polyalcohol rather than a diol as the monomer so that the polymer has a high amount of three-dimensional cross-linking. The result is a rigid but very light foam suitable for use as thermal insulation in building construction and portable ice chests.
Problem 31-8
Poly(ethylene terephthalate), or PET, is a polyester used to make soft-drink bottles. It is prepared by reaction of ethylene glycol with 1,4-benzenedicarboxylic acid (terephthalic acid). Draw the structure of PET.
Problem 31-9
Show the mechanism of the nucleophilic addition reaction of an alcohol with an isocyanate to yield a urethane.

