7 Why This Chapter?
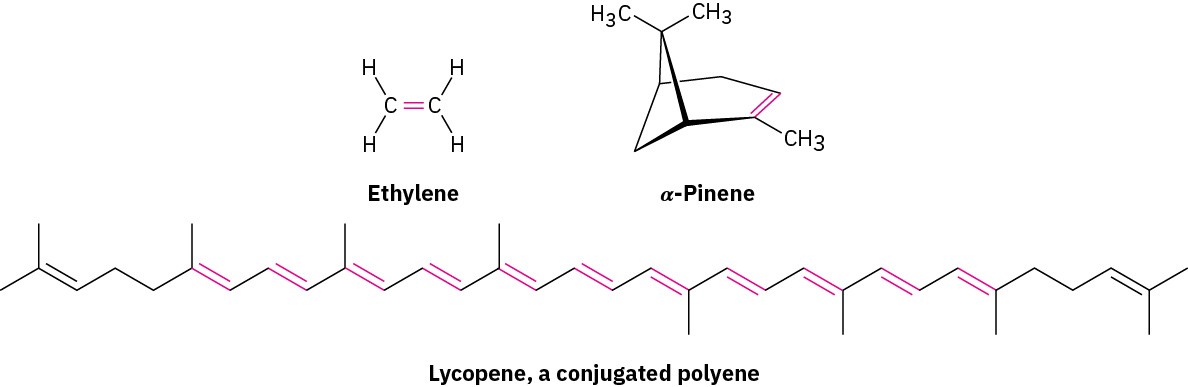
Figure 7.1 Tomatoes are good for you. Their red color is due to lycopene, which has 13 double bonds. (credit: modification of work “Tomatoes” by Jeremy Keith/Flickr, CC BY 2.0)
Carbon–carbon double bonds are present in most organic and biological molecules, so a good understanding of their behavior is needed. In this chapter, we’ll look at some consequences of alkene stereoisomerism and then focus on the broadest and most general class of alkene reactions, the electrophilic addition reaction. Carbon-carbon triple bonds, by contrast, occur much less commonly, so we’ll not spend much time on their chemistry.
An alkene, sometimes called an olefin from the German term for oil forming, is a hydrocarbon that contains a carbon–carbon double bond, while an alkyne is a hydrocarbon that contains a carbon-carbon triple bond. Alkenes occur abundantly in nature. Ethylene, for instance, is a plant hormone that induces ripening in fruit, and α– pinene is the major component of turpentine. Lycopene, found in fruits such as watermelon and papaya as well as tomatoes, is an antioxidant with numerous health benefits such as sun protection and cardiovascular protection.