23.5 Mixed Aldol Reactions
Until now, we’ve considered only symmetrical aldol reactions, in which the two carbonyl components have been the same. What would happen, though, if an aldol reaction were carried out between two different carbonyl partners?
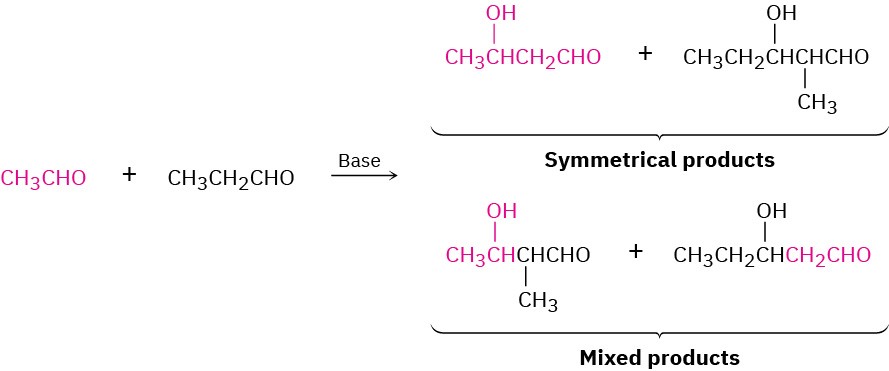
In general, a mixed aldol reaction between two similar aldehyde or ketone partners leads to a mixture of four possible products. For example, base treatment of a mixture of acetaldehyde and propanal gives a complex product mixture containing two “symmetrical” aldol products and two “mixed” aldol products. Clearly, such a reaction is of no practical value.
On the other hand, mixed aldol reactions can lead cleanly to a single product if either of two conditions is met:
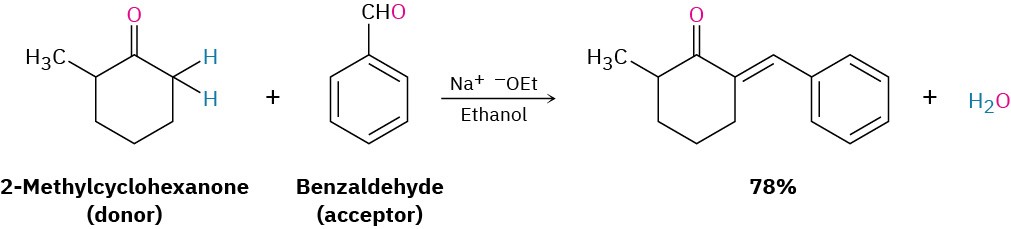
- If one of the carbonyl partners contains no α hydrogens (and thus can’t form an enolate ion to become a donor), but does contain an unhindered carbonyl group (and so is a good acceptor of nucleophiles), then a mixed aldol reaction is likely to be successful. This is the case, for instance, when either benzaldehyde or formaldehyde is used as one of the carbonyl partners.
Neither benzaldehyde nor formaldehyde can form an enolate ion to add to another partner, yet both compounds have an unhindered carbonyl group. The ketone 2- methylcyclohexanone, for instance, gives the mixed aldol product on reaction with benzaldehyde.
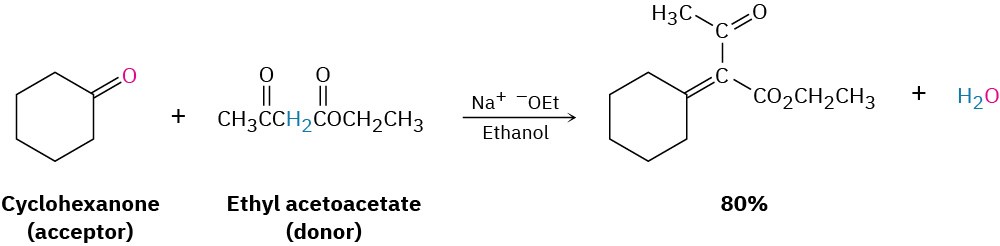
- If one of the carbonyl partners is much more acidic than the other, and is thus transformed into its enolate ion in preference to the other, then a mixed aldol reaction is likely to be successful. Ethyl acetoacetate, for instance, is completely
converted into its enolate ion in preference to enolate ion formation from monocarbonyl partners. Thus, aldol condensations of monoketones with ethyl acetoacetate occur preferentially to give the mixed product.
The situation can be summarized by saying that a mixed aldol reaction leads to a mixture of products unless one of the partners either has no α hydrogens but is a good electrophilic acceptor (such as benzaldehyde) or is an unusually acidic nucleophilic donor (such as ethyl acetoacetate).
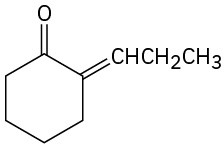
Problem 23-8
Which of the following compounds can probably be prepared by a mixed aldol reaction? Show the reactants you would use in each case.
(a)
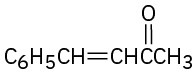
(b)
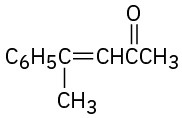
(c)