18.5 Reactions of Epoxides: Ring-Opening
Acid-Catalyzed Epoxide Opening
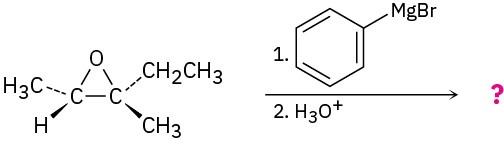
Epoxides are cleaved by treatment with acid just as other ethers are, but under much milder conditions because of ring strain. As we saw in Section 8.7, dilute aqueous acid at room temperature is sufficient for facilitating the hydrolysis of epoxides to give 1,2-diols, also called vicinal glycols. (The word vicinal means “adjacent,” and a glycol is a diol.) The epoxide cleavage takes place by SN2-like backside attack of a nucleophile on the protonated epoxide, giving a trans-1,2-diol as product.
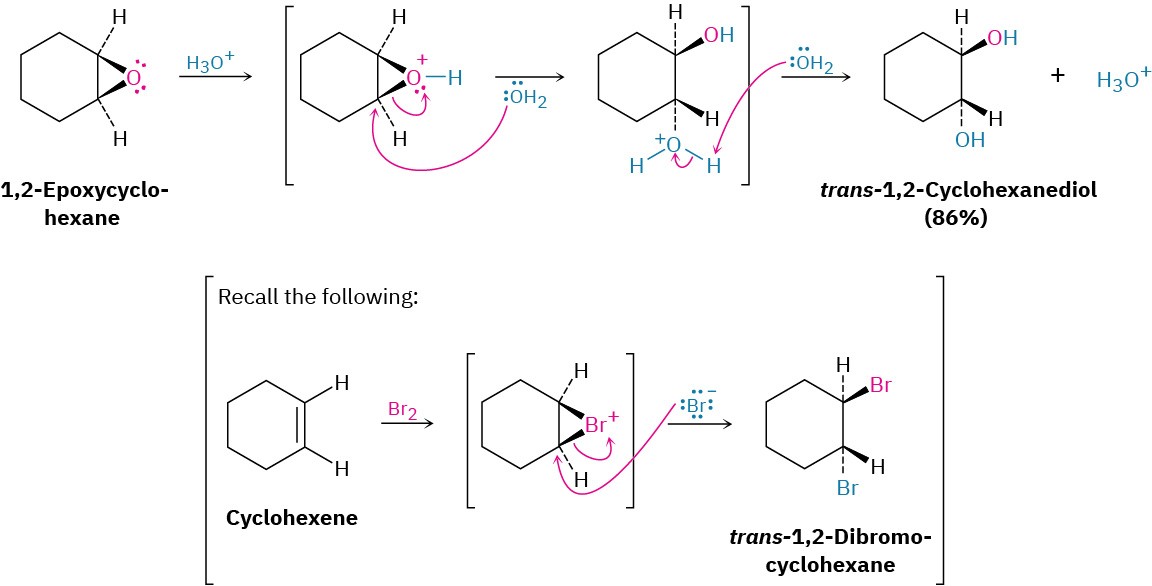
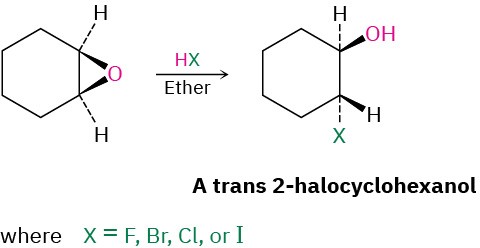
Epoxides can also be opened by reaction with acids other than H3O+. If anhydrous HX is used, for instance, an epoxide is converted into a trans halohydrin.
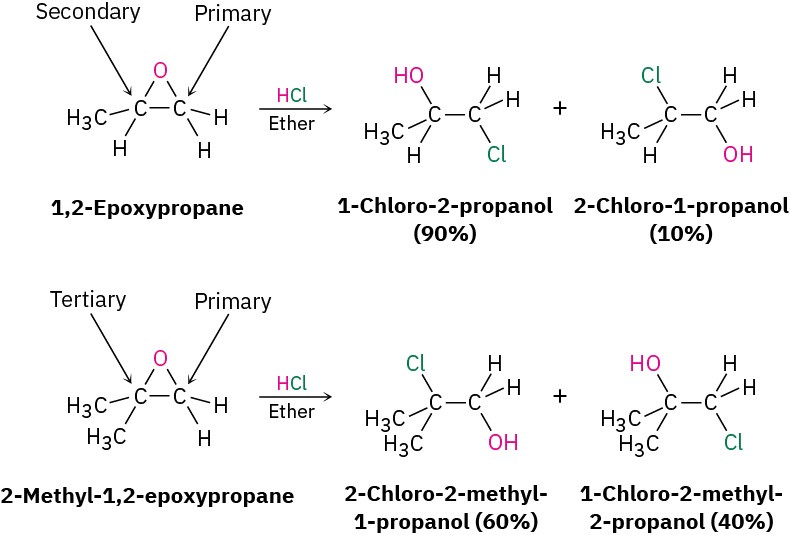
The regiochemistry of acid-catalyzed ring-opening depends on the epoxide’s structure, and a mixture of products is often formed. When both epoxide carbon atoms are either primary or secondary, attack of the nucleophile occurs primarily at the less highly substituted site: an SN2-like result. When one of the epoxide carbon atoms is tertiary, however, nucleophilic attack occurs primarily at the more highly substituted site—an SN1-like result. Thus, 1,2- epoxypropane reacts with HCl to give primarily 1-chloro-2-propanol, but 2-methyl-1,2- epoxypropane gives 2-chloro-2-methyl-1- propanol as the major product.
The mechanisms of these acid-catalyzed epoxide openings are more complex than they initially appear. They seem to be neither purely SN1 nor SN2 but instead to be midway between the two extremes and to have characteristics of both. For instance, take the reaction of 1,2-epoxy-1-methylcyclohexane with HBr, shown in Figure 18.2. The reaction yields only a single stereoisomer of 2-bromo-2-methylcyclohexanol in which the –Br and – OH groups are trans, an SN2-like result caused by backside displacement of the epoxide oxygen. But the fact that Br– attacks the more hindered tertiary side of the epoxide rather than the less hindered secondary side is an SN1-like result in which the more stable, tertiary carbocation is involved.
Evidently, the transition state for acid-catalyzed epoxide opening has an SN2-like geometry but also has a high degree of SN1-like carbocationic character. Because the positive charge in the protonated epoxide is shared by the more highly substituted carbon atom, backside attack of Br– occurs at the more highly substituted site.
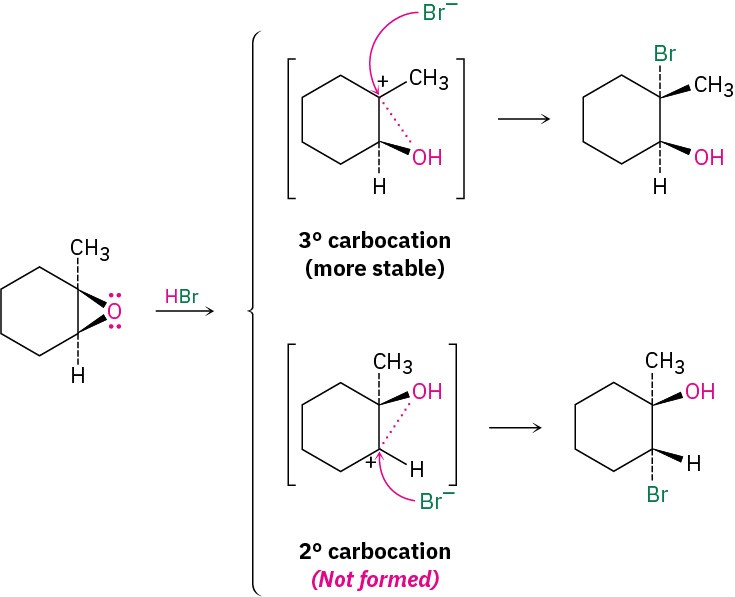
Figure 18.2 Ring-opening of 1,2-epoxy-1-methylcyclohexane with HBr. There is a high degree of SN1-like carbocation character in the transition state, which leads to backside attack of the nucleophile at the tertiary center and to formation of a product isomer that has –Br and –OH groups trans.
Worked Example 18.3Predicting the Product of Epoxide Ring-OpeningPredict the major product of the following reaction:
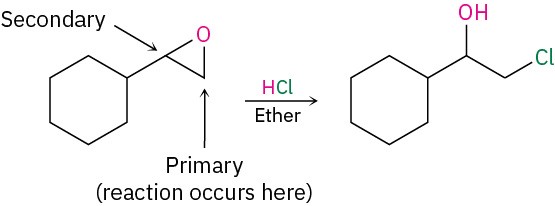
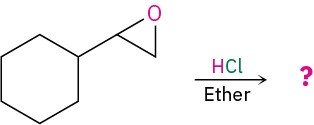 StrategyIdentify the substitution pattern of the two epoxide carbon atoms. In this case, one carbon is secondary and one is primary. Then recall the guidelines for epoxide cleavages. An epoxide with only primary and secondary carbons usually undergoes cleavage by SN2-like attack of a nucleophile on the less hindered carbon, but an epoxide with a tertiary carbon atom usually undergoes cleavage by backside attack on the more hindered carbon. In this case, an SN2 cleavage of the primary C–O epoxide bond will occur.Solution
StrategyIdentify the substitution pattern of the two epoxide carbon atoms. In this case, one carbon is secondary and one is primary. Then recall the guidelines for epoxide cleavages. An epoxide with only primary and secondary carbons usually undergoes cleavage by SN2-like attack of a nucleophile on the less hindered carbon, but an epoxide with a tertiary carbon atom usually undergoes cleavage by backside attack on the more hindered carbon. In this case, an SN2 cleavage of the primary C–O epoxide bond will occur.Solution
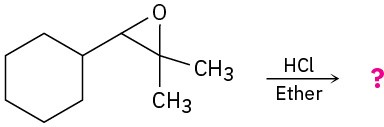
Problem 18-12
Predict the major product of each of the following reactions: (a)
(b)
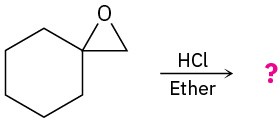
Problem 18-13
How would you prepare the following diols?
(a)
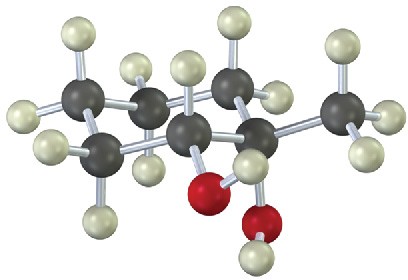
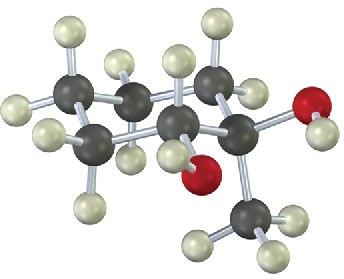
(b)
Base-Catalyzed Epoxide Opening
Unlike other ethers, epoxide rings can be cleaved by bases and nucleophiles as well as by acid. Although an ether oxygen is normally a poor leaving group in an SN2 reaction (Section 11.3), the strain of the three-membered ring causes epoxides to react with hydroxide ion at elevated temperatures.
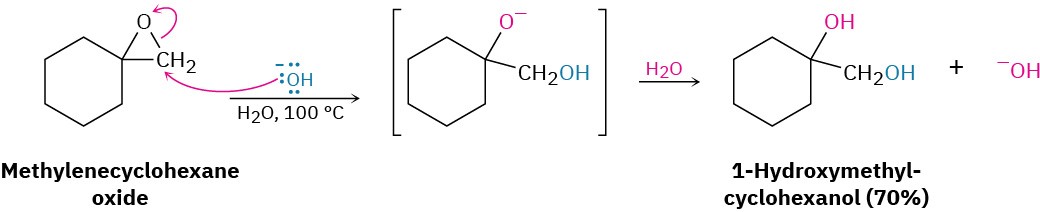
Base-catalyzed epoxide opening is a typical SN2 reaction in which attack of the nucleophile takes place at the less hindered epoxide carbon. For example, 1,2-epoxypropane reacts with ethoxide ion exclusively at the less highly substituted, primary carbon to give 1- ethoxy-2-propanol.
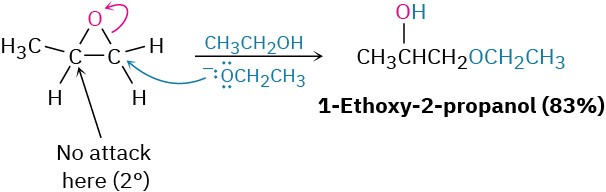
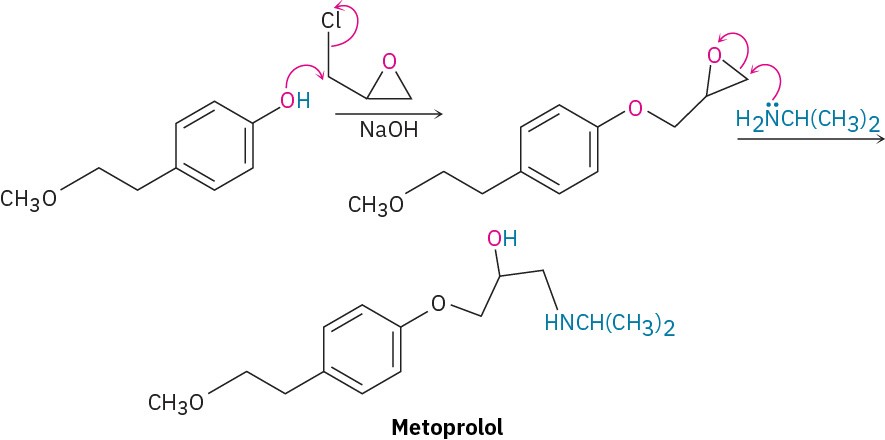
Many different nucleophiles can be used for epoxide opening, including amines (RNH2 or R2NH) and Grignard reagents (RMgX). An example of an amine reacting with an epoxide occurs in the commercial synthesis of metoprolol, a so-called β-blocker that is used for
treatment of cardiac arrhythmias, hypertension, and heart attacks. β-Blockers are among the most widely prescribed drugs in the world.
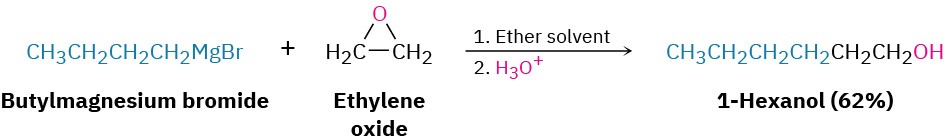
A similar nucleophilic ring-opening occurs when epoxides are treated with Grignard reagents. Ethylene oxide is frequently used, thereby allowing the conversion of a Grignard reagent into a primary alcohol having two more carbons than the starting alkyl halide. 1- Bromobutane, for example, is converted into 1-hexanol by reaction of its Grignard reagent with ethylene oxide.
Problem 18-14
Predict the major product of the following reactions: (a)
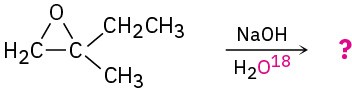
(b)
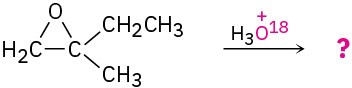
(c)