22.7 Alkylation of Enolate Ions
Perhaps the most useful reaction of enolate ions is their alkylation by treatment with an alkyl halide, thereby forming a new C−C bond and joining two smaller pieces into one larger molecule. Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide in an SN2 reaction and displaces the leaving group by backside attack.
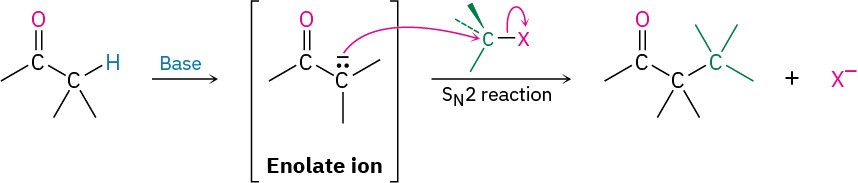
Alkylation reactions are subject to the same constraints that affect all SN2 reactions (Section 11.3). Thus, the leaving group X in the alkylating agent R−X can be chloride, bromide, or iodide. The alkyl group R should be primary or methyl, and preferably should be allylic or benzylic. Secondary halides react poorly, and tertiary halides don’t react at all because a competing E2 elimination of HX occurs instead. Vinylic and aryl halides are also unreactive because a backside approach is sterically prevented.
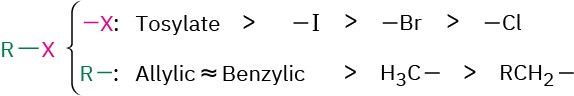
The Malonic Ester Synthesis
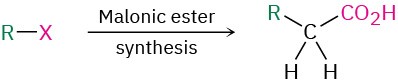
One of the oldest and best known carbonyl alkylation reactions is the malonic ester synthesis, a method for preparing a carboxylic acid from an alkyl halide while lengthening the carbon chain by two atoms.
Diethyl propanedioate, commonly called diethyl malonate, or malonic ester, is relatively acidic (pKa = 13) because its α hydrogens are flanked by two carbonyl groups. Thus, malonic ester is easily converted into its enolate ion by reaction with sodium ethoxide in ethanol. The enolate ion, in turn, is a good nucleophile that reacts rapidly with an alkyl halide to give an α-substituted malonic ester. Note in the following examples that the abbreviation “Et” is used for an ethyl group, –CH2CH3.
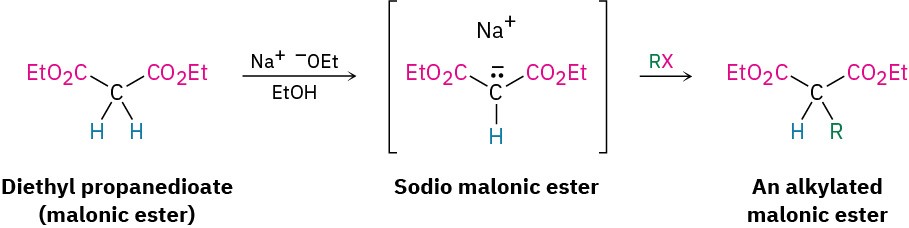
The product of a malonic ester alkylation has one acidic α hydrogen remaining, so the alkylation process can be repeated to yield a dialkylated malonic ester.
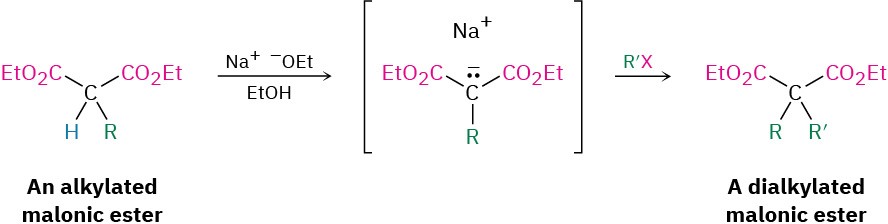
On heating with aqueous hydrochloric acid, the alkylated (or dialkylated) malonic ester undergoes hydrolysis of its two ester groups followed by decarboxylation (loss of CO2) to yield a substituted monocarboxylic acid.
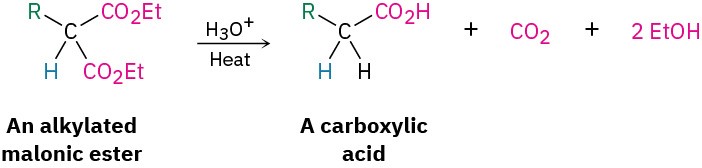
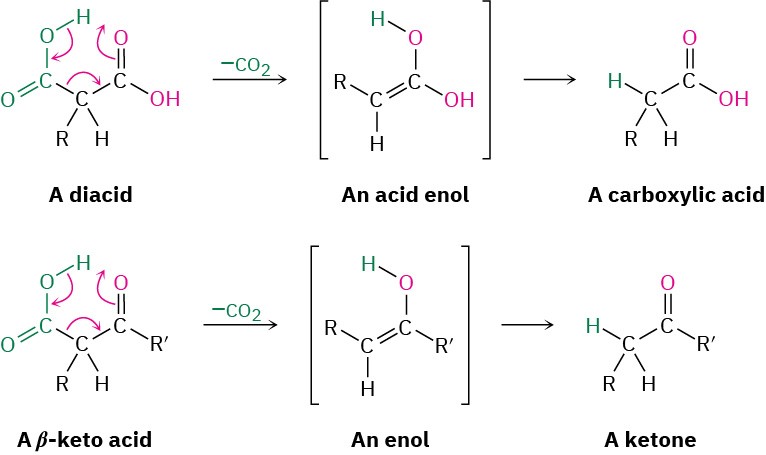
Decarboxylation is not a general reaction of carboxylic acids. Rather, it is unique to compounds that have a second carbonyl group two atoms away from the –CO2H. That is, only substituted malonic acids and β-keto acids undergo loss of CO2 on heating. The decarboxylation reaction occurs by a cyclic mechanism and involves initial formation of an enol, thereby accounting for the need to have a second carbonyl group appropriately positioned.
As noted previously, the overall effect of malonic ester synthesis is to convert an alkyl halide into a carboxylic acid while lengthening the carbon chain by two atoms
(RX → RCH2CO2H).
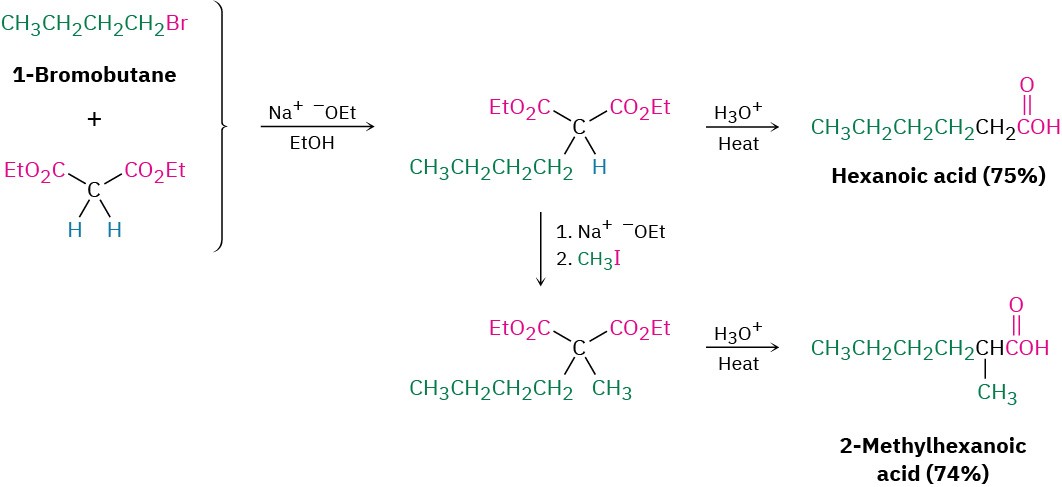
A malonic ester synthesis can also be used to prepare cycloalkanecarboxylic acids. For example, when 1,4-dibromobutane is treated with diethyl malonate in the presence of two equivalents of sodium ethoxide base, the second alkylation step occurs intramolecularly to yield a cyclic product. Hydrolysis and decarboxylation then give cyclopentanecarboxylic acid. Three-, four-, five-, and six-membered rings can be prepared in this way, but yields decrease for larger ring sizes.
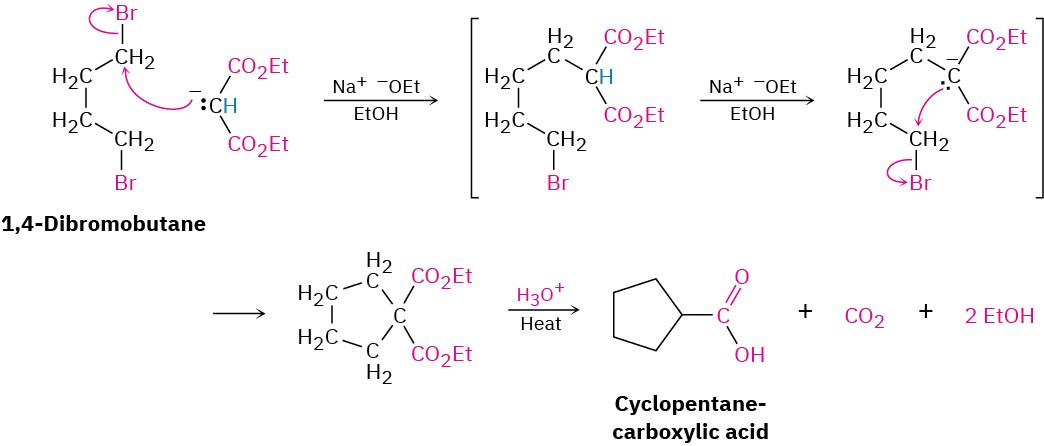 Worked Example 22.2Using Malonic Ester Synthesis to Prepare a Carboxylic AcidHow would you prepare heptanoic acid using a malonic ester synthesis?StrategyA malonic ester synthesis converts an alkyl halide into a carboxylic acid having two more carbons. Thus, a seven-carbon acid chain must be derived from the five-carbon alkyl halide 1-bromopentane.
Worked Example 22.2Using Malonic Ester Synthesis to Prepare a Carboxylic AcidHow would you prepare heptanoic acid using a malonic ester synthesis?StrategyA malonic ester synthesis converts an alkyl halide into a carboxylic acid having two more carbons. Thus, a seven-carbon acid chain must be derived from the five-carbon alkyl halide 1-bromopentane.
 Solution
Solution
Problem 22-10
How could you use a malonic ester synthesis to prepare the following compounds? Show all steps.
(a)
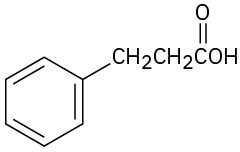
(b)
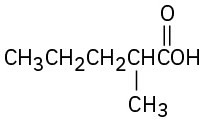
(c)
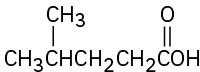
Problem 22-11
Monoalkylated and dialkylated acetic acids can be prepared by malonic ester synthesis, but trialkylated acetic acids (R3C–CO2H) can’t be prepared. Explain.
Problem 22-12
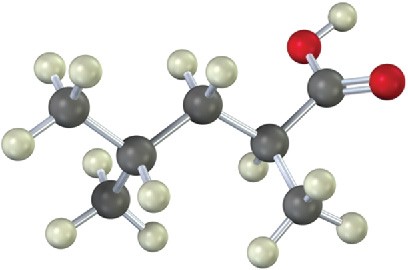
How could you use a malonic ester synthesis to prepare the following compound?
The Acetoacetic Ester Synthesis
Just as the malonic ester synthesis converts an alkyl halide into a carboxylic acid, the acetoacetic ester synthesis converts an alkyl halide into a methyl ketone having three more carbons.
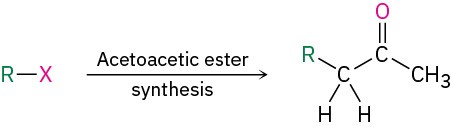
Ethyl 3-oxobutanoate, commonly called ethyl acetoacetate, or acetoacetic ester, is much like malonic ester in that its α hydrogens are flanked by two carbonyl groups. It is therefore readily converted into its enolate ion, which can be alkylated by reaction with an alkyl halide. A second alkylation can also be carried out if desired, since acetoacetic ester has two acidic α hydrogens.
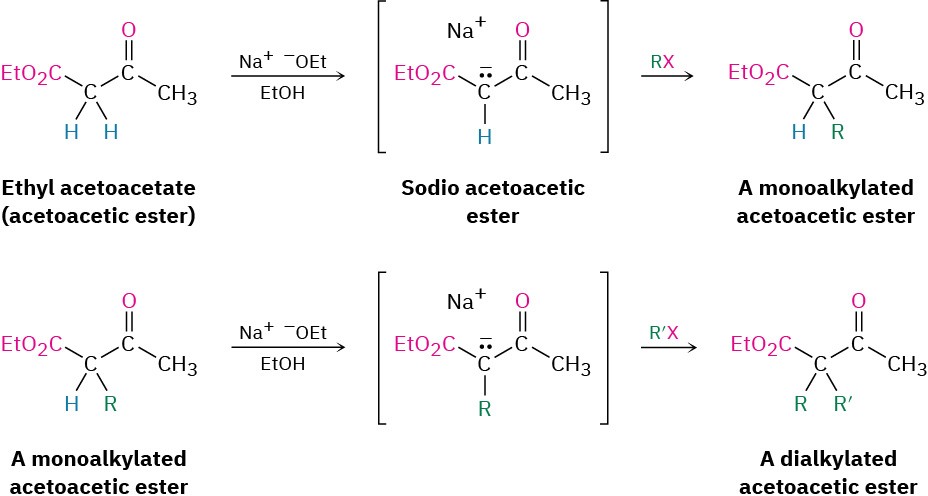
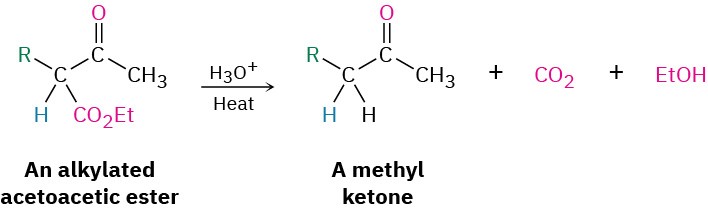
On heating with aqueous HCl, the alkylated (or dialkylated) acetoacetic ester is hydrolyzed to a β-keto acid, which then undergoes decarboxylation to yield a ketone product. The decarboxylation occurs in the same way as in the malonic ester synthesis and involves a ketone enol as the initial product.
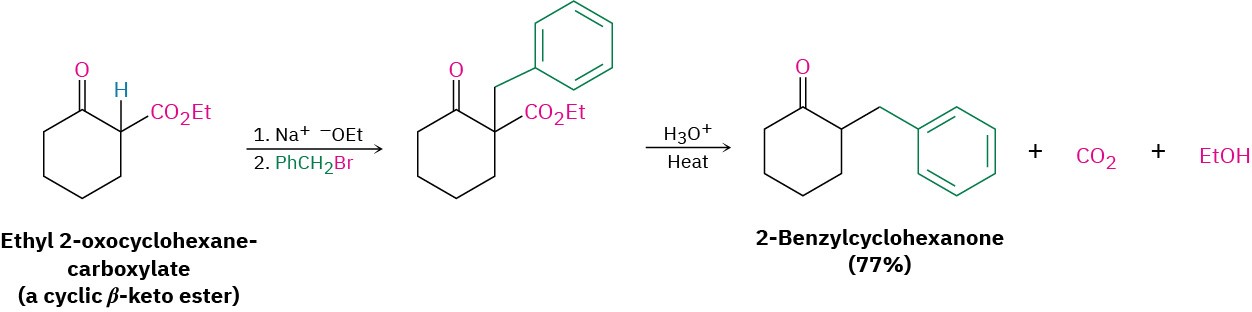
The three-step sequence of (1) enolate ion formation, (2) alkylation, and (3) hydrolysis/decarboxylation is applicable to all β-keto esters with acidic α hydrogens, not just to acetoacetic ester itself. For example, cyclic β-keto esters, such as ethyl 2- oxocyclohexanecarboxylate, can be alkylated and decarboxylated to give 2-substituted cyclohexanones.
Worked Example 22.3Using Acetoacetic Ester Synthesis to Prepare a KetoneHow would you prepare 2-pentanone by an acetoacetic ester synthesis?StrategyAn acetoacetic ester synthesis yields a methyl ketone by adding three carbons to an alkyl halide.
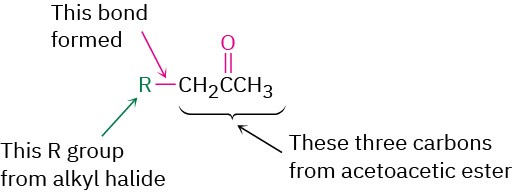
Thus, the acetoacetic ester synthesis of 2-pentanone must involve reaction of bromoethane.Solution
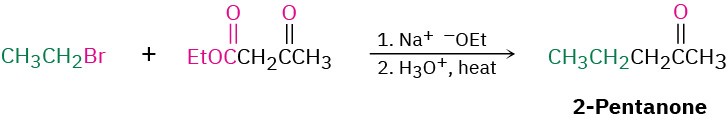
Problem 22-13
What alkyl halides would you use to prepare the following ketones by an acetoacetic ester synthesis?
(a)
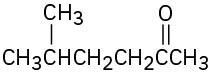
(b)
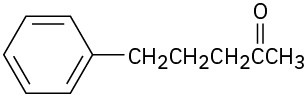
Problem 22-14
Which of the following compounds can’t be prepared by an acetoacetic ester synthesis? Explain.
(a) Phenylacetone(b) Acetophenone(c) 3,3-Dimethyl-2-butanone Problem 22-15
How would you prepare the following compound using an acetoacetic ester synthesis?
Direct Alkylation of Ketones, Esters, and Nitriles
Both the malonic ester synthesis and the acetoacetic ester synthesis are easy to carry out because they involve relatively acidic dicarbonyl compounds. As a result, sodium ethoxide in ethanol can be used to prepare the necessary enolate ions. Alternatively, however, it’s also possible in many cases to directly alkylate the α position of monocarbonyl compounds. A strong, sterically hindered base such as LDA is needed so that complete conversion to the enolate ion takes place rather than a nucleophilic addition, and a nonprotic solvent must be used.
Ketones, esters, and nitriles can all be alkylated using LDA or related dialkylamide bases in tetrahydrofuran (THF). Aldehydes, however, rarely give high yields of pure products because their enolate ions undergo carbonyl condensation reactions instead of alkylation. (We’ll study this condensation reaction in the next chapter.) Some specific examples of alkylation reactions are shown.
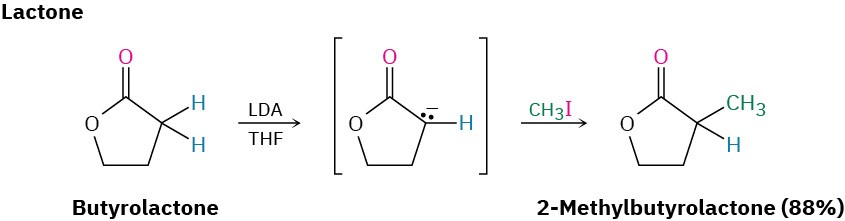
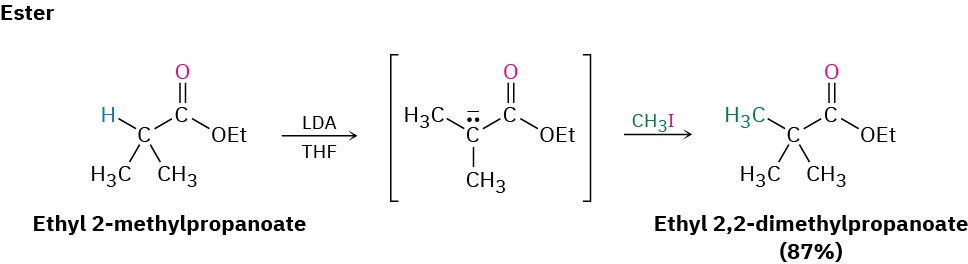
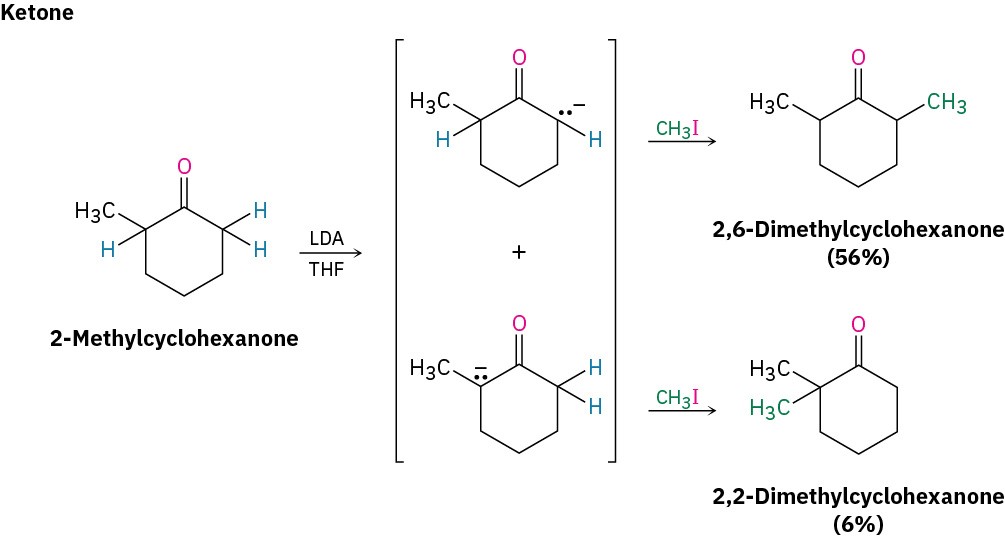
Note in the ketone example that alkylation of 2-methylcyclohexanone leads to a mixture of products because both possible enolate ions are formed. In general, the major product in such cases occurs by alkylation at the less hindered, more accessible position. Thus, alkylation of 2-methylcyclohexanone occurs primarily at C6 (secondary) rather than C2 (tertiary).
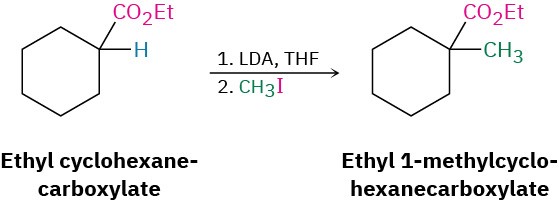
Worked Example 22.4Using an Alkylation Reaction to Prepare a Substituted Ester How might you use an alkylation reaction to prepare ethyl 1- methylcyclohexanecarboxylate?

StrategyAn alkylation reaction is used to introduce a methyl or primary alkyl group onto the α position of a ketone, ester, or nitrile by SN2 reaction of an enolate ion with an alkyl halide. Thus, we need to look at the target molecule and identify any methyl or primary alkyl groups attached to an α carbon. In the present instance, the target has an α methyl group, which might be introduced by alkylation of an ester enolate ion with iodomethane.Solution
Problem 22-16
Show how you might prepare the following compounds using an alkylation reaction as the key step:
(a)
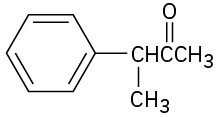
(b)
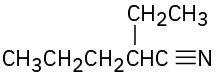
(c)
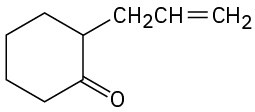
(d)
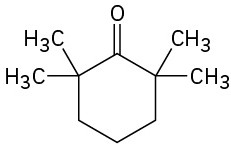
(e)
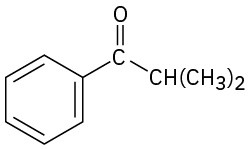
(f)
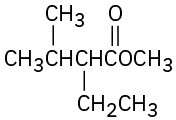
Biological Alkylations
Alkylations are rare but not unknown in biological chemistry. One example occurs during biosynthesis of the antibiotic indolmycin from indolylpyruvate when a base abstracts an acidic hydrogen from an α position and the resultant enolate ion carries out an SN2 alkylation reaction on the methyl group of S-adenosylmethionine (SAM; Section 11.6).
Although it’s convenient to speak of “enolate ion” intermediates in biological pathways, it’s unlikely that they exist for long in an aqueous cellular environment. Rather, proton removal and alkylation probably occur at essentially the same time (Figure 22.7).
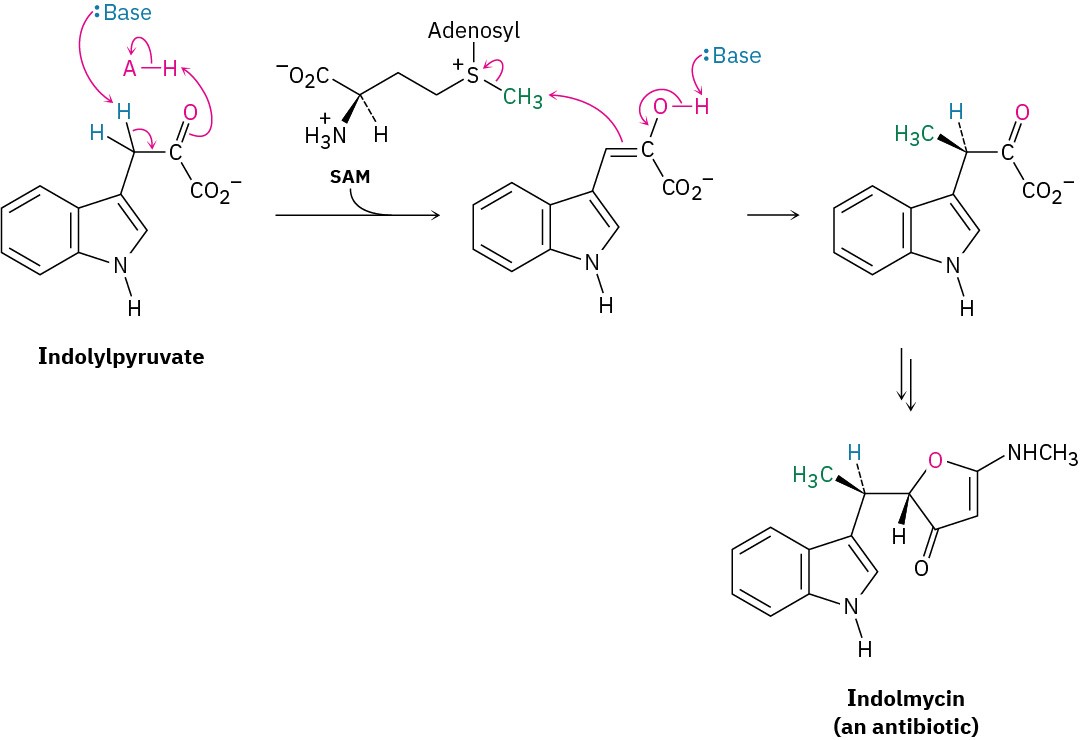
Figure 22.7 The biosynthesis of indolmycin from indolylpyruvate occurs through a pathway that includes an alkylation reaction of a short-lived enolate ion intermediate.
Additional Problems 22 • Additional Problems 22 • Additional Problems Visualizing Chemistry Problem 22-17
Show the steps in preparing each of the following substances using either a malonic ester synthesis or an acetoacetic ester synthesis:
(a)
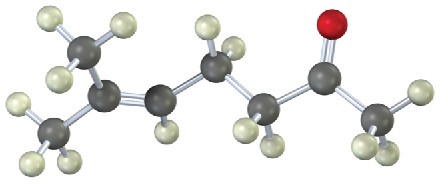
(b)
Problem 22-18
Unlike most β-diketones, the following β-diketone has no detectable enol content and is about as acidic as acetone. Explain.
Problem 22-19
For a given α hydrogen atom to be acidic, the C–H bond must be parallel to the p orbitals of the C=O double bond (that is, perpendicular to the plane of the adjacent carbonyl group). Identify the most acidic hydrogen atom in the conformation shown for the following structure. Is it axial or equatorial?
Mechanism Problems
Problem 22-20
Draw the corresponding keto or enol tautomer for each of the following molecules. (a)
(b)
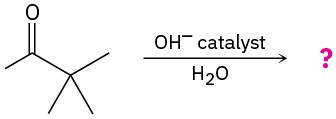
Problem 22-21
Predict the product(s) and show the mechanism for each of the following reactions: (a)
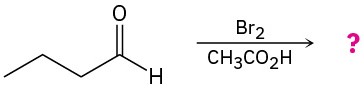
(b)
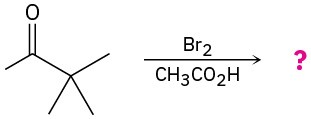
Problem 22-22
Predict the product(s) and show the mechanism for each of the following reactions: (a)
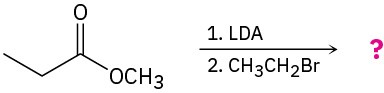
(b)
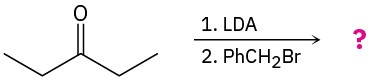
Problem 22-23
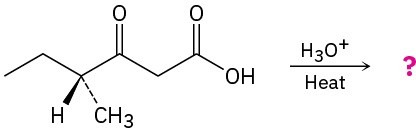
The following two optically active β-keto acids were decarboxylated under the conditions typically used for the acetoacetic ester synthesis. Will the ketone products be optically active? Explain.
(a)
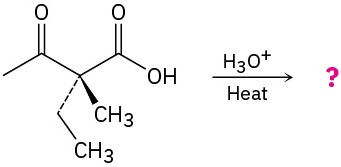
(b)
Problem 22-24
In the Hell–Volhard–Zelinskii reaction, only a catalytic amount of PBr3 is necessary because of the following equilibrium. Propose a mechanism for formation of the equilibrium.
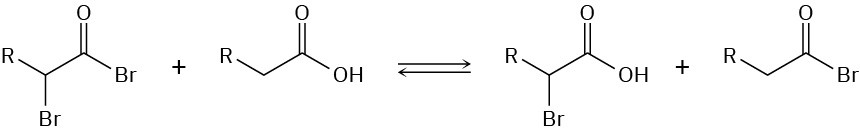
Problem 22-25
When a ketone is treated with a halogen under acidic conditions, the α-monohalogenated product can be obtained in high yield. Under basic conditions however it is difficult to isolate the monohalogenated product. Explain.
Problem 22-26
Nonconjugated β,γ-unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated α,β-unsaturated isomers. Propose a mechanism for the isomerization.
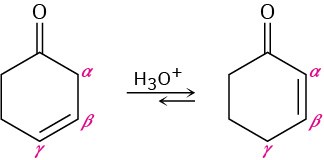
Problem 22-27
2-substituted 2-cyclopentenones can be interconverted with 5-substituted 2- cyclopentenones under basic conditions. Propose a mechanism for this isomerization.
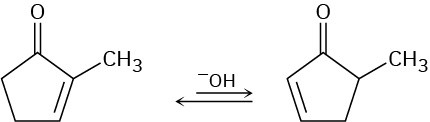
Problem 22-28
Using curved arrows, propose a mechanism for the following reaction, one of the steps in the metabolism of the amino acid alanine.
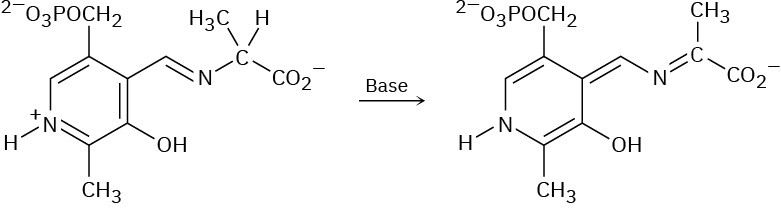
Problem 22-29
Using curved arrows, propose a mechanism for the following reaction, one of the steps in the biosynthesis of the amino acid tyrosine.
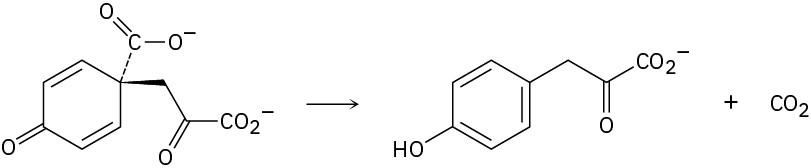
Problem 22-30
One of the later steps in glucose biosynthesis is the isomerization of fructose 6-phosphate to glucose 6-phosphate. Propose a mechanism, using acid or base catalysis as needed.
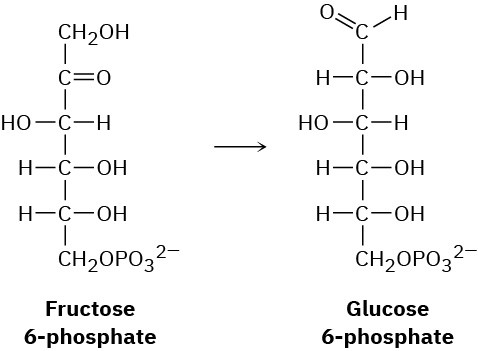
Problem 22-31
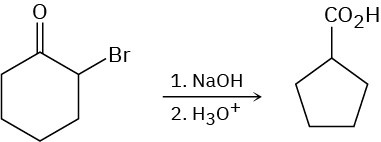
The Favorskii reaction involves treatment of an α-bromo ketone with base to yield a ring- contracted product. For example, reaction of 2-bromocyclohexanone with aqueous NaOH yields cyclopentanecarboxylic acid. Propose a mechanism.
Problem 22-32
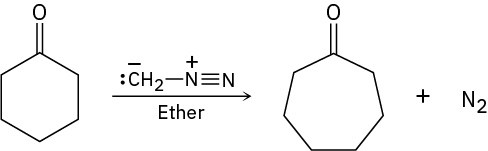
Treatment of a cyclic ketone with diazomethane is a method for accomplishing a ring- expansion reaction. For example, treatment of cyclohexanone with diazomethane yields cycloheptanone. Propose a mechanism.
Problem 22-33
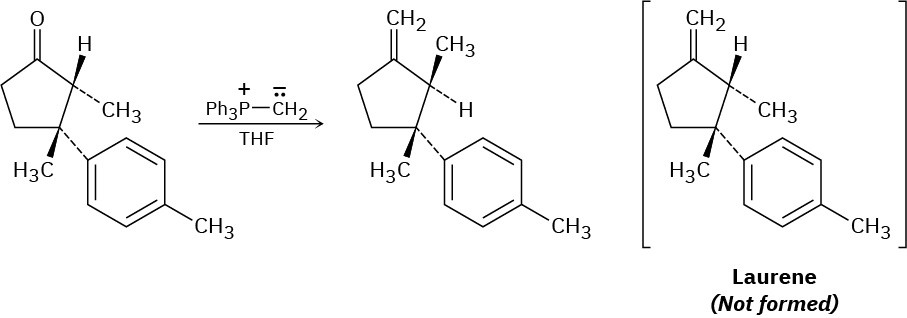
The final step in an attempted synthesis of laurene, a hydrocarbon isolated from the marine alga Laurencia glandulifera, involved the Wittig reaction shown. The product obtained, however, was not laurene but an isomer. Propose a mechanism to account for the unexpected results.
Problem 22-34
Amino acids can be prepared by reaction of alkyl halides with diethyl acetamidomalonate, followed by heating the initial alkylation product with aqueous HCl. Show how you would prepare alanine, CH3CH(NH2)CO2H, one of the twenty amino acids found in proteins, and propose a mechanism for acid-catalyzed conversion of the initial alkylation product to the amino acid.

Problem 22-35
Amino acids can also be prepared by a two-step sequence that involves Hell–Volhard– Zelinskii reaction of a carboxylic acid followed by treatment with ammonia. Show how you would prepare leucine, (CH3)2CHCH2CH(NH2)CO2H, and identify the mechanism of the second step.
Problem 22-36
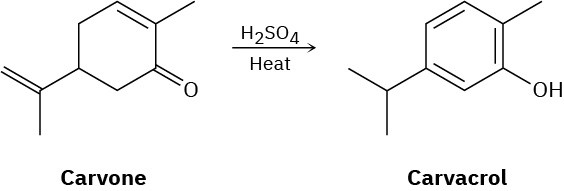
Heating carvone with aqueous sulfuric acid converts it into carvacrol. Propose a mechanism for the isomerization.
Acidity of Carbonyl Compounds
Problem 22-37
Identify all the acidic hydrogens (pKa < 25) in the following molecules: (a)
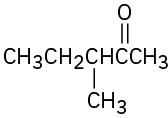
(b)
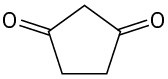
(c)
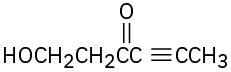
(d)
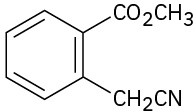
(e)
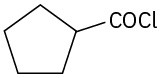
(f)
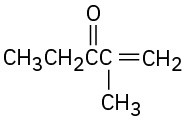
Problem 22-38
Rank the following compounds in order of increasing acidity:
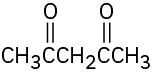 (a)
(a) 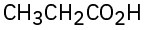 (b)
(b)  (c)
(c) 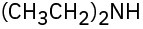 (d)
(d) 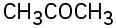 (e)(f)
(e)(f) 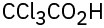
Problem 22-39
Write resonance structures for the following anions: (a)
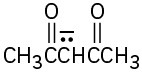
(b)
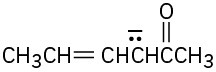
(c)
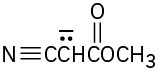
(d)
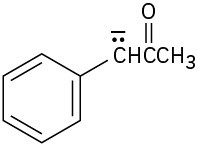
(e)
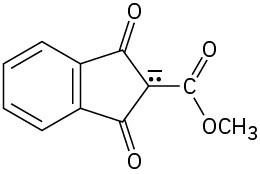
Problem 22-40
Base treatment of the following α,β-unsaturated carbonyl compound yields an anion by removal of H+ from the γ carbon. Why are hydrogens on the γ carbon atom acidic?
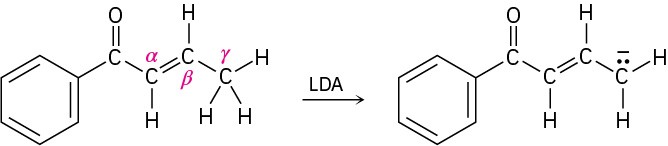
Problem 22-41
Treatment of 1-phenyl-2-propenone with a strong base such as LDA does not yield an anion, even though it contains a hydrogen on the carbon atom next to the carbonyl group. Explain.
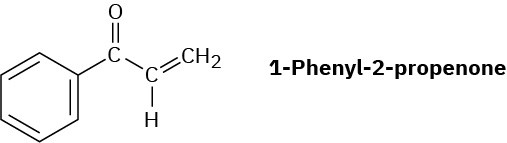
α-Substitution Reactions
Problem 22-42
Predict the product(s) of the following reactions:
(a)
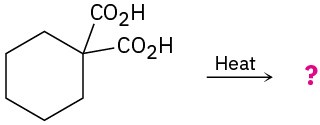
(b)
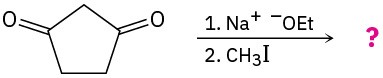
(c)
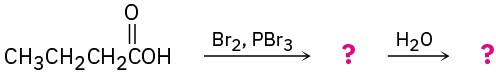
(d)
Problem 22-43
Which, if any, of the following compounds can be prepared by a malonic ester synthesis? Show the alkyl halide you would use in each case.
(a) Ethyl pentanoate(b) Ethyl 3-methylbutanoate(c) Ethyl 2-methylbutanoate
(d) Ethyl 2,2-dimethylpropanoate Problem 22-44
Which, if any, of the following compounds can be prepared by an acetoacetic ester synthesis? Explain.
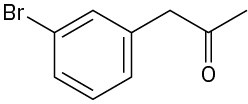 (a)(b)
(a)(b) 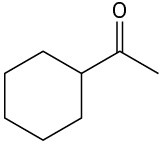 (c)
(c) 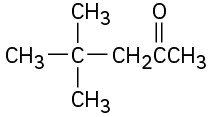
Problem 22-45
How would you prepare the following ketones using an acetoacetic ester synthesis? (a)
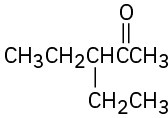
(b)
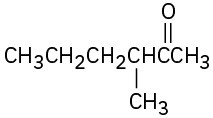
Problem 22-46
How would you prepare the following compounds using either an acetoacetic ester synthesis or a malonic ester synthesis?
(a)
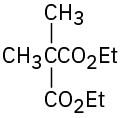
(b)
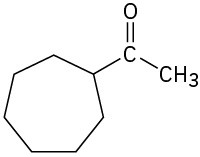
(c)
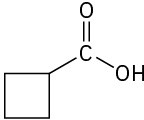
(d)
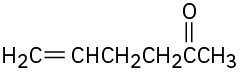
Problem 22-47
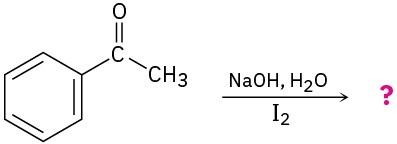
Which of the following substances would undergo the haloform reaction?
(a) CH3COCH3(b) Acetophenone(c) CH3CH2CHO(d) CH3CO2H(e) CH3C≡N Problem 22-48
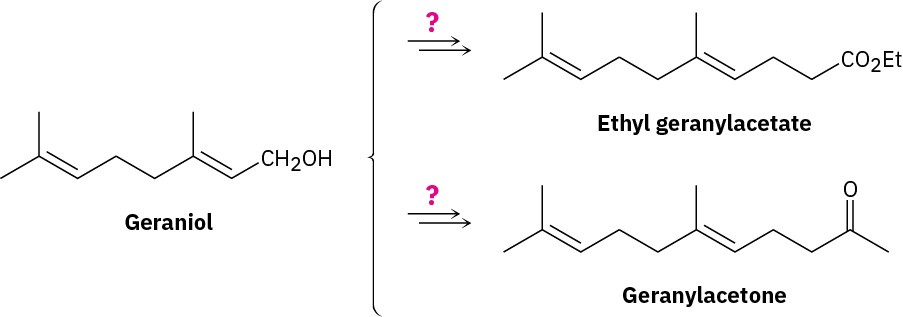
How might you convert geraniol into either ethyl geranylacetate or geranylacetone?
Problem 22-49
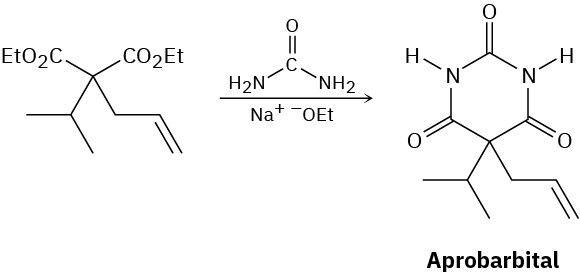
Aprobarbital, a barbiturate once used in treating insomnia, is synthesized in three steps from diethyl malonate. Show how you would synthesize the necessary dialkylated intermediate, and then propose a mechanism for the reaction of this intermediate with urea to give aprobarbital.
General Problems
Problem 22-50
One way to determine the number of acidic hydrogens in a molecule is to treat the compound with NaOD in D2O, isolate the product, and determine its molecular weight by mass spectrometry. For example, if cyclohexanone is treated with NaOD in D2O, the product has MW = 102. Explain how this method works.
Problem 22-51
When optically active (R)-2-methylcyclohexanone is treated with either aqueous base or acid, racemization occurs. Explain.
Problem 22-52
Would you expect optically active (S)-3-methylcyclohexanone to be racemized on acid or base treatment in the same way as 2-methylcyclohexanone (Problem 22-51)? Explain.
Problem 22-53
When an optically active carboxylic acid such as (R)-2-phenylpropanoic acid is brominated under Hell–Volhard–Zelinskii conditions, is the product optically active or racemic?
Explain.
Problem 22-54
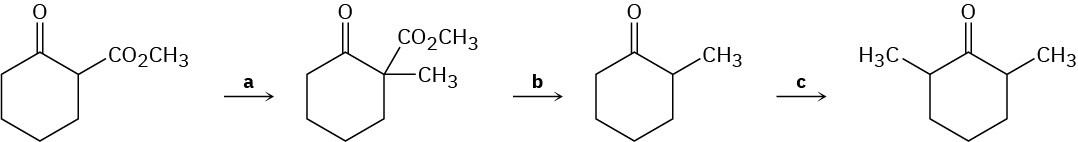
Fill in the reagents a–c that are missing from the following scheme:
Problem 22-55
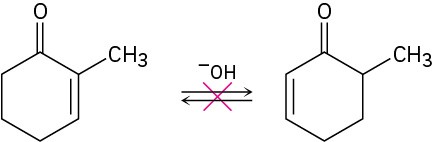
Although 2-substituted 2-cyclopentenones are in a base-catalyzed equilibrium with their 5- substituted 2-cyclopentenone isomers, the analogous isomerization is not observed for 2- substituted 2-cyclohexenones. Explain.
Problem 22-56
How would you synthesize the following compounds from cyclohexanone? More than one step may be required.
(a)
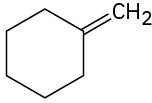
(b)
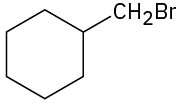
(c)
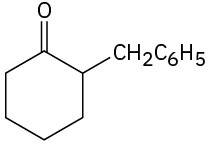
(d)
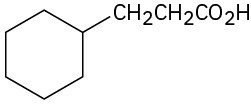
(e)
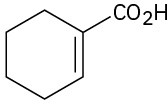
(f)
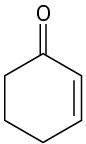
Problem 22-57
The two isomers cis– and trans-4-tert-butyl-2-methylcyclohexanone are interconverted by base treatment. Which isomer do you think is more stable, and why?
Problem 22-58
The following synthetic routes are incorrect. What is wrong with each? (a)
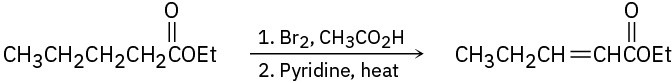
(b)
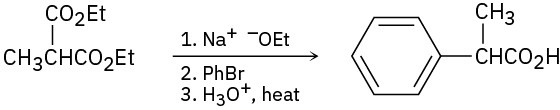
(c)
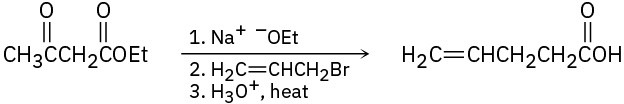
Problem 22-59
Attempted Grignard reaction of cyclohexanone with tert-butylmagnesium bromide yields only about 1% of the expected addition product along with 99% unreacted cyclohexanone. If D3O+ is added to the reaction mixture after a suitable period, however, the “unreacted” cyclohexanone is found to have one deuterium atom incorporated into it. Explain.
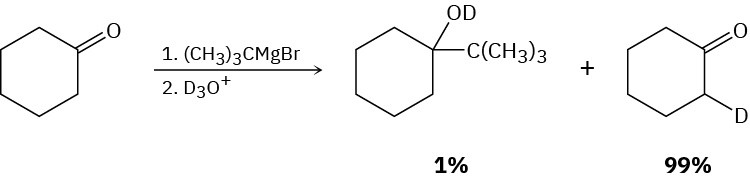
Problem 22-60
Ketones react slowly with benzeneselenenyl chloride in the presence of HCl to yield α– phenylseleno ketones. Propose a mechanism for this acid-catalyzed α-substitution reaction.
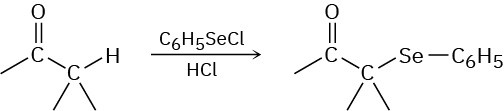
Problem 22-61
South American Incas chewed the leaves of the coca bush, Erythroxylon coca, to combat fatigue. Chemical studies of Erythroxylon coca by Friedrich Wöhler in 1862 resulted in the discovery of cocaine, C17H21NO4, as the active component. Basic hydrolysis of cocaine leads to methanol, benzoic acid, and another compound called ecgonine, C9H15NO3. Oxidation of ecgonine with CrO3 yields a keto acid that readily loses CO2 on heating, giving tropinone.
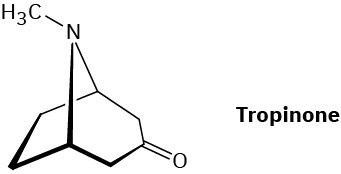
(a)
What is a likely structure for the keto acid? (b)
What is a likely structure for ecgonine, neglecting stereochemistry? (c)
What is a likely structure for cocaine, neglecting stereochemistry? Problem 22-62
The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?
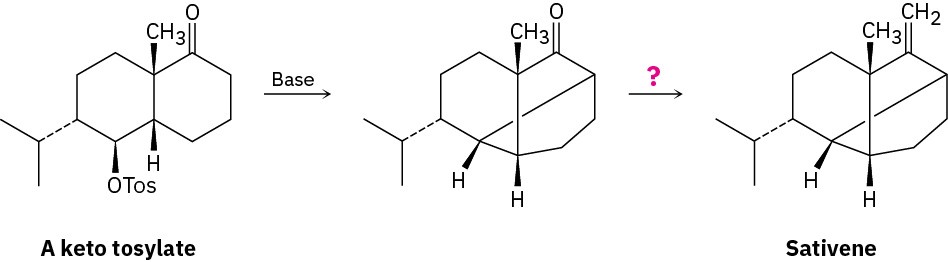
Problem 22-63
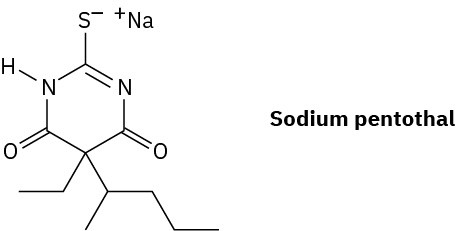
Sodium pentothal is a short-acting barbiturate derivative used as a general anesthetic and known in popular culture as a truth serum. It is synthesized like other barbiturates (see the Chemistry Matters at the end of this chapter), using thiourea, (H2N)2C═S, in place of urea.
How would you synthesize sodium pentothal?