5.1 Enantiomers and the Tetrahedral Carbon
What causes molecular handedness? Look at generalized molecules of the type CH3X, CH2XY, and CHXYZ shown in Figure 5.2. On the left are three molecules, and on the right are their images reflected in a mirror. The CH3X and CH2XY molecules are identical to their mirror images and thus are not handed. If you make a molecular model of each molecule and its mirror image, you find that you can superimpose one on the other so that all atoms coincide. The CHXYZ molecule, by contrast, is not identical to its mirror image. You can’t superimpose a model of this molecule on a model of its mirror image for the same reason that you can’t superimpose a left hand on a right hand: they simply aren’t the same.
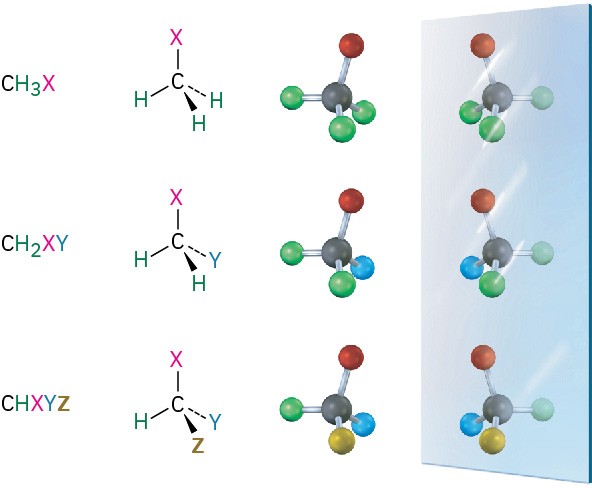
Figure 5.2 Tetrahedral carbon atoms and their mirror images. Molecules of the type CH3X and CH2XY are identical to their mirror images, but a molecule of the type CHXYZ is not. A CHXYZ molecule is related to its mirror image in the same way a right hand is related to a left hand.
A molecule that is not identical to its mirror image is a kind of stereoisomer (Section 4.2) called an enantiomer (e-nan-tee-oh-mer, from the Greek enantio, meaning “opposite”). Enantiomers are related to each other as a right hand is related to a left hand and result whenever a tetrahedral carbon is bonded to four different substituents (one need not be H). For example, lactic acid (2-hydroxypropanoic acid) exists as a pair of enantiomers because there are four different groups (−H, −OH, −CH3, −CO2H) bonded to the central carbon atom. The enantiomers are called (+)-lactic acid and (−)-lactic acid. Both are found in sour milk, but only the (+) enantiomer occurs in muscle tissue.
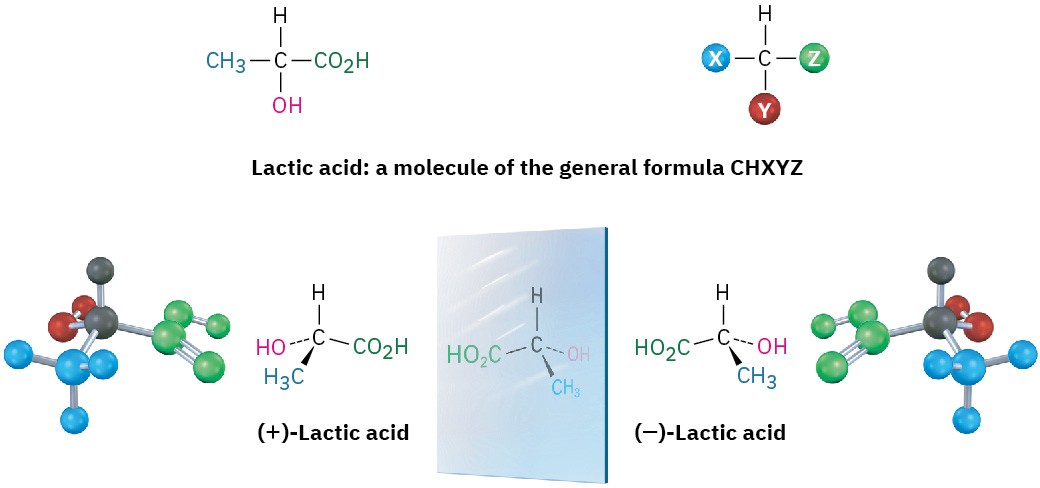
No matter how hard you try, you can’t superimpose a molecule of (+)-lactic acid on a molecule of (−)-lactic acid. If any two groups match up, say −H and −CO2H, the remaining two groups don’t match (Figure 5.3).
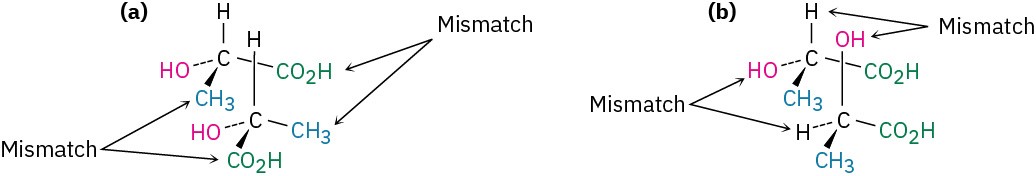
Figure 5.3 Attempts at superimposing the mirror-image forms of lactic acid. (a) When the −H and −OH substituents match up, the −CO2H and −CH3 substituents don’t; (b) when −CO2H and −CH3 match up, −H and −OH don’t. Regardless of how the molecules are oriented, they aren’t identical.

